International Journal of Hydrogen Energy ( IF 8.1 ) Pub Date : 2023-06-26 , DOI: 10.1016/j.ijhydene.2023.06.100 Dogan Kaya , Ilker Demiroglu , Ilknur Baldan Isik , Hasan Huseyin Isik , Selda Kılıç Çetin , Cem Sevik , Ahmet Ekicibil , Faruk Karadag
|
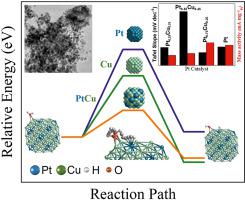
Improving the activity and stability of Pt-based electrocatalysts is still crucial for hydrogen generation applications. In this study, we investigated the catalytic properties of Cu-doped Pt alloy nanostructures synthesized by the modified polyol method, and compared the performance of PtCu catalyst with that of commercially available Pt/C for the hydrogen evolution reaction (HER) at room temperature. Structural analyses revealed that the PtCu catalysts exhibited fcc- crystal structure with an average particle size below 5 nm. The HER performance of the catalysts showed overpotential of around −1.0 V (vs. Ag/AgCl) and Pt0.25Cu0.75 and Pt0.75Cu0.25 catalysts exhibited enhanced performance in 1 M KOH. The Pt0.75Cu0.25 catalyst exhibited distinct performance, with the highest mass activity found to be 62.80 mA mg−1Pt. The most active Pt0.75Cu0.25 catalysis for the HER process had the lowest onset potential of 0.989 V and Tafel slope of 35.5 mV dec−1, which were an improvement compared to commercially available Pt/C catalysts. First principle DFT calculations confirmed the stabilities of Pt1Cu3, Pt1Cu1 and Pt3Cu1 compositions as ordered alloy structures. Nudged elastic band method calculations and the d-band model verified the higher catalytic activity of Pt–Cu nanoparticles compared to pure nanoparticles and point out the relevance of a synergistic effect of Pt and Cu atoms on water dissociation. According to DFT calculations, Cu and Pt sites significantly influenced the adsorption of H2O and H, respectively, for splitting water molecules. While the Pt59Cu20 cluster has the highest H2O adsorption energy at the (100) atop site of Cu atoms, H adsorption occurs at the (111) site of Pt atoms.
中文翻译:

用于析氢反应电催化的高活性双金属 Pt-Cu 纳米粒子:实验和理论见解
提高铂基电催化剂的活性和稳定性对于制氢应用仍然至关重要。在本研究中,我们研究了采用改良多元醇法合成的Cu掺杂Pt合金纳米结构的催化性能,并将PtCu催化剂与市售Pt/C催化剂在室温析氢反应(HER)中的性能进行了比较。结构分析表明 PtCu 催化剂表现出 fcc-平均粒径小于5纳米的晶体结构。催化剂的 HER 性能显示出约 -1.0 V(相对于 Ag/AgCl)的过电势,并且 Pt 0.25 Cu 0.75和 Pt 0.75 Cu 0.25催化剂在 1 M KOH 中表现出增强的性能。Pt 0.75 Cu 0.25催化剂表现出独特的性能,最高质量活性为62.80 mA mg -1 Pt。HER 过程中最活跃的 Pt 0.75 Cu 0.25催化具有最低的起始电位 0.989 V 和塔菲尔斜率 35.5 mV dec -1,与市售 Pt/C 催化剂相比,这是一项改进。第一原理DFT计算证实了Pt 1 Cu 3、Pt 1 Cu 1和Pt 3 Cu 1组合物作为有序合金结构的稳定性。微移弹性带法计算和 d 带模型验证了 Pt-Cu纳米颗粒与纯纳米颗粒相比具有更高的催化活性,并指出了Pt 和 Cu 原子对水解离的协同效应的相关性。根据DFT计算,Cu和Pt位点显着影响H 2的吸附O和H分别用于分裂水分子。Pt 59 Cu 20簇在Cu原子的(100)位点处具有最高的H 2 O吸附能,而H吸附发生在Pt原子的(111)位点处。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号