Acta Biomaterialia ( IF 9.4 ) Pub Date : 2023-06-24 , DOI: 10.1016/j.actbio.2023.06.024
Ting Zhao , Bokai Gong , Shiqin Luo , Rongping Zhang , Ling Zhang , Yuan Huang , Huile Gao , Tao Gong
|
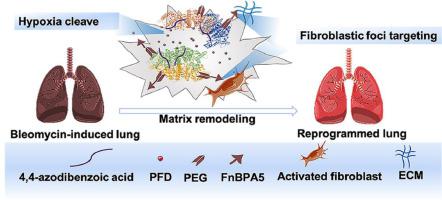
The progressive formation of fibroblastic foci characterizes idiopathic pulmonary fibrosis (IPF), and excessive oral doses of approved pirfenidone (PFD) always cause gastrointestinal side effects. The fibrotic response driven by activated fibroblasts could perpetuate epithelial damage and promote abnormal extracellular matrix (ECM) deposition. When modified nanoparticles reach their target, it is important to ensure a responsive release of PFD. Hypoxia is a determining factor in IPF, leading to alveolar dysfunction and deeper cellular fibrosis. Herein, a fibroblastic foci-targeting and hypoxia-cleavable drug delivery system (Fn-Azo-BSA@PEG) was established to reprogram the fibrosis in IPF. We have modified the FnBAP5 peptide to enable comprehensive fibroblastic foci targeting, which helps BSA nanoparticles recognize and accumulate at fibrotic sites. Meantime, the hypoxia-responsive azobenzene group allowed for efficient and rapid drug diffusion, while the PEGylated BSA reduced system toxicity and increased circulation in vivo. As expected, the strategy of the fibronectin-targeting-modification and hypoxia-responsive drug release synergistically inhibited activated fibroblasts and reduced the secretion of the fibrosis-related protein. Fn-Azo-BSA@PEG could accumulate in pulmonary tissue and prolong the survival time in bleomycin-induced pulmonary fibrosis mice. Together, the multivalent BSA nanoparticles offered an efficient approach for improving lung architecture and function by regulating the fibroblastic foci and hypoxia.
Statement of significance
We established fibroblastic foci-targeting and hypoxia-cleavable bovine serum albumin (BSA) nanoparticles (Fn-Azo-BSA@PEG) to reprogramme the fibroblastic foci in idiopathic pulmonary fibrosis (IPF). Fn-Azo-BSA@PEG was designed to actively target fibroblasts and abnormal ECM with the FnBPA5 peptide, delivering more FDA-approved pirfenidone (PFD) to the cross-talk within the foci. Once the drug reached fibroblastic foci, the azobenzene group acted as a hypoxia-responsive linker to trigger effective and rapid drug release. Hypoxic responsiveness and FnBAP5-modification of Fn-Azo-BSA@PEG synergistically inhibited the secretion of proteins closely related to fibrogenesis. BSA's inherent transport and metabolic pathways in the pulmonary reduced the side effects of the main organs. The multivalent BSA nanoparticles efficiently inhibited IPF-fibrosis progress and preserved the lung architecture by regulating the fibroblastic foci and hypoxia.
中文翻译:

用于治疗特发性肺纤维化的成纤维细胞病灶靶向和缺氧可裂解的吡非尼酮递送系统
成纤维细胞灶的进行性形成是特发性肺纤维化(IPF)的特征,过量口服已批准的吡非尼酮(PFD)总是会引起胃肠道副作用。由活化的成纤维细胞驱动的纤维化反应可以使上皮损伤永久化并促进异常的细胞外基质(ECM)沉积。当修饰纳米粒子时达到目标后,确保 PFD 的快速发布非常重要。缺氧是 IPF 的决定因素,导致肺泡功能障碍和更深的细胞纤维化。在此,建立了一种成纤维细胞病灶靶向和缺氧可裂解的药物递送系统(Fn-Azo-BSA@PEG)来重新编程IPF中的纤维化。我们对 FnBAP5 肽进行了修饰,以实现全面的成纤维细胞病灶靶向,这有助于 BSA 纳米颗粒在纤维化部位识别和积累。同时,缺氧响应性偶氮苯基团允许有效和快速的药物扩散,而聚乙二醇化 BSA 降低了系统毒性并增加了体内。正如预期的那样,纤连蛋白靶向修饰和缺氧响应药物释放的策略协同抑制了活化的成纤维细胞并减少了纤维化相关蛋白的分泌。Fn-Azo-BSA@PEG可以在博来霉素诱导的肺纤维化小鼠的肺组织中积累并延长其存活时间。多价 BSA 纳米粒子共同提供了一种通过调节成纤维细胞灶和缺氧来改善肺结构和功能的有效方法。
重要性声明
我们建立了成纤维细胞病灶靶向和缺氧可裂解的牛血清白蛋白(BSA)纳米颗粒(Fn-Azo-BSA@PEG)来重新编程特发性肺纤维化(IPF)中的成纤维细胞病灶。Fn-Azo-BSA@PEG 旨在利用 FnBPA5 肽主动靶向成纤维细胞和异常 ECM,为病灶内的串扰提供更多 FDA 批准的吡非尼酮 (PFD)。一旦药物到达成纤维细胞灶,偶氮苯基团就会充当缺氧反应性连接基,从而触发有效且快速的药物释放。Fn-Azo-BSA@PEG 的缺氧反应性和 FnBAP5 修饰可协同抑制与纤维形成密切相关的蛋白质的分泌。BSA 在肺部固有的运输和代谢途径减少了对主要器官的副作用。































 京公网安备 11010802027423号
京公网安备 11010802027423号