当前位置:
X-MOL 学术
›
Chem. Biodivers.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Synthesis, Antifungal Activity, and Molecular Simulation Study of L–Carvone-Derived 1,3,4-Oxadiazole-Thioether Compounds
Chemistry & Biodiversity ( IF 2.3 ) Pub Date : 2023-06-29 , DOI: 10.1002/cbdv.202300794 Rongzhu Wen 1, 2 , Wengui Duan 1, 2 , Guishan Lin 1, 2 , Baoyu Li 1, 2 , Zhaolei Zhang 1, 2 , Chuwen Liu 1, 2
Chemistry & Biodiversity ( IF 2.3 ) Pub Date : 2023-06-29 , DOI: 10.1002/cbdv.202300794 Rongzhu Wen 1, 2 , Wengui Duan 1, 2 , Guishan Lin 1, 2 , Baoyu Li 1, 2 , Zhaolei Zhang 1, 2 , Chuwen Liu 1, 2
Affiliation
|
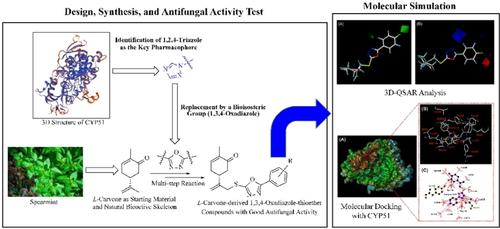
To discover potent antifungal molecules with new and distinctive structures, 20 novel L-carvone-derived 1,3,4-oxadiazole-thioether compounds 5 a–5 t were synthesized through multi-step reaction of L-carvone, and their structures were confirmed by FT-IR, 1H-NMR, 13C-NMR, and HR-MS. The antifungal activities of compounds 5 a–5 t were preliminarily tested by in vitro method, and the results indicated that all of the title compounds displayed certain antifungal activities against the eight tested plant fungi, especially for P. piricola. Among them, compound 5 i (R=p-F) with the most significant antifungal activity deserved further study for discovering and developing novel natural product-based antifungal agents. Moreover, two molecular simulation technologies were employed for the investigation of their structure–activity relationships (SARs). Firstly, a reasonable and effective 3D-QSAR model was established by the comparative molecular field (CoMFA) method, and the relationship of the substituents linked with the benzene rings and the inhibitory activities of the title compounds against P. piricola was elucidated. Then, the binding mode of compound 5 i (R=p-F) and its potential biological target (CYP51) was simulated by molecular docking, and it was found that compound 5 i could readily bind with CYP51 in the active site, and the ligand-receptor interactions involved three hydrogen bonds and several hydrophobic effects.
中文翻译:

L-香芹酮衍生的 1,3,4-恶二唑-硫醚化合物的合成、抗真菌活性和分子模拟研究
为了发现具有新的、独特结构的强效抗真菌分子,通过L-香芹酮的多步反应合成了20种新型的L-香芹酮衍生的1,3,4-恶二唑硫醚化合物5a – 5t,并确认了它们的结构通过 FT-IR、1 H-NMR、13 C-NMR 和 HR-MS。采用体外方法初步测试了化合物5a ~ 5t的抗真菌活性,结果表明,标题化合物对8种受试植物真菌均表现出一定的抗真菌活性,特别是对P. piricola。其中,化合物5i(R= p- F)具有最显着的抗真菌活性,值得进一步研究,以发现和开发新型天然产物抗真菌剂。此外,还采用了两种分子模拟技术来研究它们的构效关系(SAR)。首先,采用比较分子场(CoMFA)方法建立了合理有效的3D-QSAR模型,阐明了苯环上连接的取代基与标题化合物对P. piricola抑制活性的关系。然后,通过分子对接模拟了化合物5i(R= p- F)与其潜在生物靶点(CYP51)的结合模式,发现化合物5i可以很容易地与CYP51的活性位点结合,并且配体-受体相互作用涉及三个氢键和几种疏水效应。
更新日期:2023-06-29
中文翻译:

L-香芹酮衍生的 1,3,4-恶二唑-硫醚化合物的合成、抗真菌活性和分子模拟研究
为了发现具有新的、独特结构的强效抗真菌分子,通过L-香芹酮的多步反应合成了20种新型的L-香芹酮衍生的1,3,4-恶二唑硫醚化合物5a – 5t,并确认了它们的结构通过 FT-IR、1 H-NMR、13 C-NMR 和 HR-MS。采用体外方法初步测试了化合物5a ~ 5t的抗真菌活性,结果表明,标题化合物对8种受试植物真菌均表现出一定的抗真菌活性,特别是对P. piricola。其中,化合物5i(R= p- F)具有最显着的抗真菌活性,值得进一步研究,以发现和开发新型天然产物抗真菌剂。此外,还采用了两种分子模拟技术来研究它们的构效关系(SAR)。首先,采用比较分子场(CoMFA)方法建立了合理有效的3D-QSAR模型,阐明了苯环上连接的取代基与标题化合物对P. piricola抑制活性的关系。然后,通过分子对接模拟了化合物5i(R= p- F)与其潜在生物靶点(CYP51)的结合模式,发现化合物5i可以很容易地与CYP51的活性位点结合,并且配体-受体相互作用涉及三个氢键和几种疏水效应。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号