当前位置:
X-MOL 学术
›
J. Label. Comp. Radiopharm.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Carbon 14 synthesis of glycine transporter 1 inhibitor Iclepertin (BI 425809) and its major metabolites
Journal of Labelled Compounds and Radiopharmaceuticals ( IF 0.9 ) Pub Date : 2023-06-29 , DOI: 10.1002/jlcr.4051
Bachir Latli 1 , Matt J Hrapchak 1 , Rogelio P Frutos 1 , Heewon Lee 1 , Jinhua J Song 1
Journal of Labelled Compounds and Radiopharmaceuticals ( IF 0.9 ) Pub Date : 2023-06-29 , DOI: 10.1002/jlcr.4051
Bachir Latli 1 , Matt J Hrapchak 1 , Rogelio P Frutos 1 , Heewon Lee 1 , Jinhua J Song 1
Affiliation
|
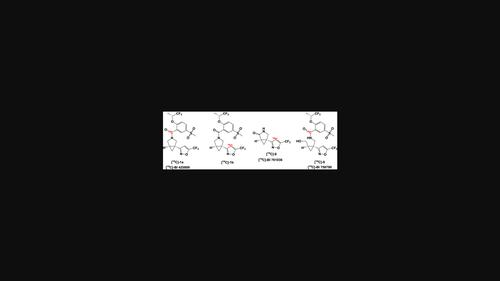
Carbon 14 labeled Iclepertin (BI 425809, 1) and its major metabolites were needed for ADME and several other studies necessary for the advancement of this drug candidate in clinical trials. Iclepertin is composed of two main chemical blocks, (R)-5-(methylsulfonyl)-2-([1,1,1-trifluoropropan-2-yl]oxy)benzoic acid (2), and 3-[(1R,5R)-3-azabicyclo[3.1.0]hexan-5-yl]-5-(trifluoromethyl)isoxazole (3) linked to each other via an amide bond. In the first synthesis of carbon 14 labeled 1, 2-fluorobenzoic acid, carboxyl-14C was converted to [14C]-2 in three steps and then coupled to 3 to provide [14C]-1a in 45% overall yield. In the second synthesis, [14C]-3 was prepared in six radioactive steps and coupled to the acid 2 to furnish [14C]-1b in 20% overall yield. Both synthetic routes provided [14C]-1a and [14C]-1b with specific activities higher than 53 mCi/mmol and radiochemical, chemical, and enantiomeric purities above 98%. Two major metabolites of 1, BI 761036 and BI 758790, were also prepared labeled with carbon 14 using intermediates already available from the synthesis of [14C]-1.
中文翻译:

甘氨酸转运蛋白 1 抑制剂 Iclepertin (BI 425809) 及其主要代谢物的碳 14 合成
ADME 和其他几项研究需要碳 14 标记的Iclepertin ( BI 425809 , 1 ) 及其主要代谢物,以推动该候选药物在临床试验中的进展。Iclepertin 由两个主要化学嵌段组成,( R )-5-(甲磺酰基)-2-([1,1,1-三氟丙-2-基]氧基)苯甲酸( 2 ) 和 3-[(1 R ,5R ) -3-氮杂双环[3.1.0]己-5-基]-5-(三氟甲基)异恶唑( 3 )通过酰胺键彼此连接。在碳14标记的1,2-氟苯甲酸的第一次合成中,羧基- 14 C分三步转化为[ 14 C]-2 ,然后与3偶联以提供[ 14 C]-1a ,总产率为45%。在第二次合成中,[ 14 C]-3通过六个放射性步骤制备,并与酸2偶联,以 20% 的总产率提供[ 14 C]-1b 。两条合成路线均提供了比活度高于53 mCi/mmol的[ 14 C]-1a和[ 14 C]-1b,以及放射化学、化学和对映体纯度高于98%。1的两种主要代谢物BI 761036和BI 758790也使用已从[ 14 C]-1合成中获得的中间体制备成碳14 标记。
更新日期:2023-06-29
中文翻译:

甘氨酸转运蛋白 1 抑制剂 Iclepertin (BI 425809) 及其主要代谢物的碳 14 合成
ADME 和其他几项研究需要碳 14 标记的Iclepertin ( BI 425809 , 1 ) 及其主要代谢物,以推动该候选药物在临床试验中的进展。Iclepertin 由两个主要化学嵌段组成,( R )-5-(甲磺酰基)-2-([1,1,1-三氟丙-2-基]氧基)苯甲酸( 2 ) 和 3-[(1 R ,5R ) -3-氮杂双环[3.1.0]己-5-基]-5-(三氟甲基)异恶唑( 3 )通过酰胺键彼此连接。在碳14标记的1,2-氟苯甲酸的第一次合成中,羧基- 14 C分三步转化为[ 14 C]-2 ,然后与3偶联以提供[ 14 C]-1a ,总产率为45%。在第二次合成中,[ 14 C]-3通过六个放射性步骤制备,并与酸2偶联,以 20% 的总产率提供[ 14 C]-1b 。两条合成路线均提供了比活度高于53 mCi/mmol的[ 14 C]-1a和[ 14 C]-1b,以及放射化学、化学和对映体纯度高于98%。1的两种主要代谢物BI 761036和BI 758790也使用已从[ 14 C]-1合成中获得的中间体制备成碳14 标记。

































 京公网安备 11010802027423号
京公网安备 11010802027423号