客观的
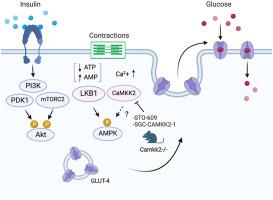
AMP 激活蛋白激酶 (AMPK) 会因收缩等能量应激而被激活,并在调节各种代谢过程(例如骨骼肌中不依赖于胰岛素的葡萄糖摄取)中发挥着至关重要的作用。通过骨骼肌中 α-AMPK Thr172 磷酸化激活 AMPK 的主要上游激酶是 LKB1,但一些研究表明 Ca 2+ /钙调蛋白依赖性蛋白激酶激酶 2 (CaMKK2) 可作为激活 AMPK 的替代激酶。我们的目的是确定 CaMKK2 是否参与骨骼肌收缩后 AMPK 的激活和促进葡萄糖摄取。
方法
使用最近开发的 CaMKK2 抑制剂 (SGC-CAMKK2-1) 以及结构相关但无活性的化合物 (SGC-CAMKK2-1N) 以及 CaMKK2 敲除 (KO) 小鼠。进行了 CaMKK 抑制剂(STO-609 和 SGC-CAMKK2-1)的体外激酶抑制选择性和功效测定以及细胞抑制功效分析。评估了用/不用 CaMKK 抑制剂处理或从野生型 (WT)/CaMKK2 KO 小鼠中分离的小鼠骨骼肌收缩后(离体)AMPK 的磷酸化和活性。通过 qPCR 测量小鼠组织中的Camkk2 mRNA。CaMKK2 蛋白表达通过预先富集或不富集骨骼肌提取物中的钙调蛋白结合蛋白的免疫印迹以及基于小鼠骨骼肌和 C2C12 肌管的基于质谱的蛋白质组学来评估。
结果
在无细胞和基于细胞的测定中,STO-609 和 SGC-CAMKK2-1 在抑制 CaMKK2 方面同样有效,但 SGC-CAMKK2-1 的选择性更高。收缩刺激的 AMPK 磷酸化和激活不受 CaMKK 抑制剂或 CaMKK2 缺失肌肉的影响。WT 肌肉和 CaMKK2 KO 肌肉之间收缩刺激的葡萄糖摄取相当。CaMKK 抑制剂(STO-609 和 SGC-CAMKK2-1)和非活性化合物(SGC-CAMKK2-1N)均显着抑制收缩刺激的葡萄糖摄取。SGC-CAMKK2-1 还抑制药理学 AMPK 激活剂或胰岛素诱导的葡萄糖摄取。在小鼠骨骼肌中检测到相对较低水平的Camkk2 mRNA,但在小鼠骨骼肌组织中未检测到 CaMKK2 蛋白及其衍生肽。
结论
我们证明 CaMKK2 的药理学抑制或遗传缺失不会影响收缩刺激的 AMPK 磷酸化和激活,以及骨骼肌中的葡萄糖摄取。之前观察到的 STO-609 对 AMPK 活性和葡萄糖摄取的抑制作用可能是由于脱靶效应所致。CaMKK2 蛋白要么不存在于成年小鼠骨骼肌中,要么低于当前可用方法的检测限。
 "点击查看英文标题和摘要"
"点击查看英文标题和摘要"
CaMKK2 is not involved in contraction-stimulated AMPK activation and glucose uptake in skeletal muscle
Objective
The AMP-activated protein kinase (AMPK) gets activated in response to energetic stress such as contractions and plays a vital role in regulating various metabolic processes such as insulin-independent glucose uptake in skeletal muscle. The main upstream kinase that activates AMPK through phosphorylation of α-AMPK Thr172 in skeletal muscle is LKB1, however some studies have suggested that Ca2+/calmodulin-dependent protein kinase kinase 2 (CaMKK2) acts as an alternative kinase to activate AMPK. We aimed to establish whether CaMKK2 is involved in activation of AMPK and promotion of glucose uptake following contractions in skeletal muscle.
Methods
A recently developed CaMKK2 inhibitor (SGC-CAMKK2-1) alongside a structurally related but inactive compound (SGC-CAMKK2-1N), as well as CaMKK2 knock-out (KO) mice were used. In vitro kinase inhibition selectivity and efficacy assays, as well as cellular inhibition efficacy analyses of CaMKK inhibitors (STO-609 and SGC-CAMKK2-1) were performed. Phosphorylation and activity of AMPK following contractions (ex vivo) in mouse skeletal muscles treated with/without CaMKK inhibitors or isolated from wild-type (WT)/CaMKK2 KO mice were assessed. Camkk2 mRNA in mouse tissues was measured by qPCR. CaMKK2 protein expression was assessed by immunoblotting with or without prior enrichment of calmodulin-binding proteins from skeletal muscle extracts, as well as by mass spectrometry-based proteomics of mouse skeletal muscle and C2C12 myotubes.
Results
STO-609 and SGC-CAMKK2-1 were equally potent and effective in inhibiting CaMKK2 in cell-free and cell-based assays, but SGC-CAMKK2-1 was much more selective. Contraction-stimulated phosphorylation and activation of AMPK were not affected with CaMKK inhibitors or in CaMKK2 null muscles. Contraction-stimulated glucose uptake was comparable between WT and CaMKK2 KO muscle. Both CaMKK inhibitors (STO-609 and SGC-CAMKK2-1) and the inactive compound (SGC-CAMKK2-1N) significantly inhibited contraction-stimulated glucose uptake. SGC-CAMKK2-1 also inhibited glucose uptake induced by a pharmacological AMPK activator or insulin. Relatively low levels of Camkk2 mRNA were detected in mouse skeletal muscle, but neither CaMKK2 protein nor its derived peptides were detectable in mouse skeletal muscle tissue.
Conclusions
We demonstrate that pharmacological inhibition or genetic loss of CaMKK2 does not affect contraction-stimulated AMPK phosphorylation and activation, as well as glucose uptake in skeletal muscle. Previously observed inhibitory effect of STO-609 on AMPK activity and glucose uptake is likely due to off-target effects. CaMKK2 protein is either absent from adult murine skeletal muscle or below the detection limit of currently available methods.






















































 京公网安备 11010802027423号
京公网安备 11010802027423号