Journal of Molecular Structure ( IF 4.0 ) Pub Date : 2023-06-28 , DOI: 10.1016/j.molstruc.2023.136111
Yongtong Lei , Jinglong Zhao , Haiming Song , Fanhang Yang , Luli Shen , Lijing Zhu , Zhixiang Zeng , Xiaocheng Li , Gang Wang
|
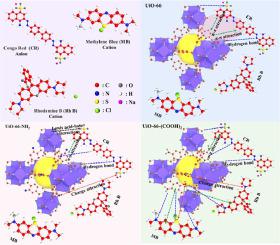
UiO-66-based metal-organic framework (MOF) materials are a class of porous crystalline materials consisting of Zr metal clusters compounded with organic ligands of terephthalic acid group. In this study, functionalized UiO-66-X (X=H, NH2, (COOH)2) nanoparticles were synthesized. The morphology of UiO-66-X nanoparticles were characterized by SEM, while the crystal structure was analyzed using XRD and TGA. Chemical composition of UiO-66-X nanoparticles were analyzed through TFIR and XPS. The adsorption capacities of UiO-66-X nanoparticles for anionic dye of Congo red (CR), and cationic dyes of methylene blue (MB) and rhodamine B (Rh B) dyes were evaluated. Furthermore, the adsorption mechanisms of UiO-66-X nanoparticles for dyes were analyzed and summarized. Results showed that the adsorption capacities of UiO-66 and UiO-66-NH2 on CR were 93.05 mg/g and 94.20 mg/g, respectively. In particular, the adsorption capacities of UiO-66-(COOH)2 on MB and Rh B were significantly increased with the adsorption capacities of 80.66 mg/g and 72.64 mg/g, respectively. Due to the strong π-π effect and hydrogen bonding interactions between UiO-66 and CR dyes. UiO-66-NH2 has stronger hydrophilicity and has electrostatic interactions, Lewis acid-base interactions and hydrogen bonds with CR. The abundant -COOH groups and higher negative Zeta potential (-23.43 mV) in UiO-66-(COOH)2 led to strong electrostatic interactions and Lewis acid-base interactions with the cationic dyes MB and Rh B. Therefore, the adsorption performance of UiO-66-(COOH)2 on cationic dyes was improved.
中文翻译:

功能化 UiO-66 纳米颗粒增强染料吸附:吸附特性和机制
UiO-66基金属有机骨架(MOF)材料是一类由Zr金属簇与对苯二甲酸基有机配体复合而成的多孔晶体材料。在本研究中,合成了功能化UiO-66-X ( X =H , NH 2 , (COOH) 2 ) 纳米粒子。通过SEM对UiO-66-X纳米粒子的形貌进行表征,同时使用XRD和TGA分析晶体结构。通过 TFIR 和 XPS 分析了 UiO-66-X 纳米颗粒的化学成分。UiO-66-X纳米颗粒对阴离子染料刚果红(CR)、阳离子染料亚甲基蓝(MB)和对罗丹明 B (Rh B) 染料进行了评估。此外,还分析总结了UiO-66-X纳米颗粒对染料的吸附机理。结果表明,UiO-66和UiO-66-NH 2对CR的吸附容量分别为93.05 mg/g和94.20 mg/g。特别是UiO-66-(COOH) 2对MB和Rh B的吸附容量显着增加,吸附容量分别为80.66 mg/g和72.64 mg/g。由于UiO-66和CR染料之间强烈的π-π效应和氢键相互作用。UiO-66-NH 2具有较强的亲水性,并与CR具有静电相互作用、路易斯酸碱相互作用以及氢键。丰富的-COOH基团和较高的负电荷UiO-66-(COOH) 2中的Zeta电位(-23.43 mV )导致与阳离子染料MB和Rh B产生强烈的静电相互作用和路易斯酸碱相互作用。因此,UiO-66-(COOH) 2 的吸附性能对阳离子染料的染色性能得到了改善。

































 京公网安备 11010802027423号
京公网安备 11010802027423号