当前位置:
X-MOL 学术
›
J. Inorg. Biochem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Mobilization of iron stored in bacterioferritin, a new target for perturbing iron homeostasis and developing antibacterial and antibiofilm molecules
Journal of Inorganic Biochemistry ( IF 3.8 ) Pub Date : 2023-06-26 , DOI: 10.1016/j.jinorgbio.2023.112306 Mario Rivera 1
Journal of Inorganic Biochemistry ( IF 3.8 ) Pub Date : 2023-06-26 , DOI: 10.1016/j.jinorgbio.2023.112306 Mario Rivera 1
Affiliation
|
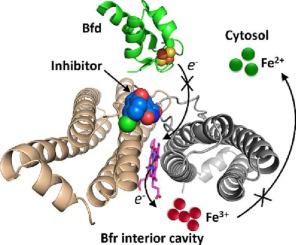
Antibiotic resistance is a global public health threat. The care of chronic infections is complicated by bacterial biofilms. Biofilm embedded cells can be up to 1000-fold more tolerant to antibiotic treatment than planktonic cells. Antibiotic tolerance is a condition which does not involve mutation and enables bacteria to survive in the presence of antibiotics. The antibiotic tolerance of biofilm-cells often renders antibiotics ineffective, even against strains that do not carry resistance-impairing mutations. This review discusses bacterial iron homeostasis and the strategies being developed to target this bacterial vulnerability, with emphasis on a recently proposed approach which aims at targeting the iron storage protein bacterioferritin (Bfr) and its physiological partner, the ferredoxin Bfd. Bfr regulates cytosolic iron concentrations by oxidizing Fe and storing Fe in its internal cavity, and by forming a complex with Bfd to reduce Fe in the internal cavity and release Fe to the cytosol. Blocking the Bfr-Bfd complex in cells causes an irreversible accumulation of Fe in BfrB and simultaneous cytosolic iron depletion, which leads to impaired biofilm maintenance and biofilm cell death. Recently discovered small molecule inhibitors of the Bfr-Bfd complex, which bind Bfr at the Bfd binding site, inhibit iron mobilization, and elicit biofilm cell death.
中文翻译:

动员储存在细菌铁蛋白中的铁,这是扰乱铁稳态和开发抗菌和抗生物膜分子的新靶点
抗生素耐药性是一个全球性的公共卫生威胁。细菌生物膜使慢性感染的护理变得复杂。生物膜包埋的细胞对抗生素治疗的耐受性比浮游细胞高 1000 倍。抗生素耐受是一种不涉及突变并使细菌能够在抗生素存在下存活的情况。生物膜细胞的抗生素耐受性通常使抗生素无效,即使对不携带耐药性损害突变的菌株也是如此。本综述讨论了细菌铁稳态和为解决这种细菌脆弱性而开发的策略,重点介绍了最近提出的一种方法,该方法旨在靶向铁储存蛋白细菌铁蛋白 (Bfr) 及其生理伙伴铁氧还蛋白 Bfd。Bfr 通过氧化 Fe 并将 Fe 储存在其内腔中,并通过与 Bfd 形成复合物来降低内腔中的 Fe 并将 Fe 释放到胞质溶胶中,从而调节胞质铁浓度。 阻断细胞中的 Bfr-Bfd 复合物会导致 Fe 在 BfrB 中不可逆地积累,同时胞质铁耗竭,从而导致生物膜维持受损和生物膜细胞死亡。最近发现的 Bfr-Bfd 复合物的小分子抑制剂,它在 Bfd 结合位点结合 Bfr,抑制铁动员,并引发生物膜细胞死亡。
更新日期:2023-06-26
中文翻译:

动员储存在细菌铁蛋白中的铁,这是扰乱铁稳态和开发抗菌和抗生物膜分子的新靶点
抗生素耐药性是一个全球性的公共卫生威胁。细菌生物膜使慢性感染的护理变得复杂。生物膜包埋的细胞对抗生素治疗的耐受性比浮游细胞高 1000 倍。抗生素耐受是一种不涉及突变并使细菌能够在抗生素存在下存活的情况。生物膜细胞的抗生素耐受性通常使抗生素无效,即使对不携带耐药性损害突变的菌株也是如此。本综述讨论了细菌铁稳态和为解决这种细菌脆弱性而开发的策略,重点介绍了最近提出的一种方法,该方法旨在靶向铁储存蛋白细菌铁蛋白 (Bfr) 及其生理伙伴铁氧还蛋白 Bfd。Bfr 通过氧化 Fe 并将 Fe 储存在其内腔中,并通过与 Bfd 形成复合物来降低内腔中的 Fe 并将 Fe 释放到胞质溶胶中,从而调节胞质铁浓度。 阻断细胞中的 Bfr-Bfd 复合物会导致 Fe 在 BfrB 中不可逆地积累,同时胞质铁耗竭,从而导致生物膜维持受损和生物膜细胞死亡。最近发现的 Bfr-Bfd 复合物的小分子抑制剂,它在 Bfd 结合位点结合 Bfr,抑制铁动员,并引发生物膜细胞死亡。































 京公网安备 11010802027423号
京公网安备 11010802027423号