Chemical Engineering Journal ( IF 13.3 ) Pub Date : 2023-06-24 , DOI: 10.1016/j.cej.2023.144190 Ke Zhu , Wenlei Qin , Yaping Gan , Yizhe Huang , Zhiwei Jiang , Yuwen Chen , Xin Li , Kai Yan
|
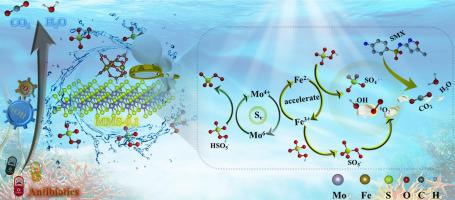
Iron-based molybdenum disulfide (Fe-MoS2) has emerged as a Fenton-like catalyst for the highly efficient degradation of antibiotics, but the structure–activity relationship remains elusive. Herein, garland-like MIL-101(Fe)/MoS2 nanosheets (MMS) with dual metal active sites (Fe and Mo) and rich sulfur vacancies were fabricated to directly activate peroxymonosulfate (PMS) for fast degradation of different organic pollutants (phenols, dyes and drugs), even in real water bodies. The MMS exhibited extremely fast catalytic rate constant of 0.289 min−1 in the degradation of sulfamethoxazole (SMX), which was about 36 and 29 times that of single MoS2 (0.008 min−1) and MIL-101(Fe) (0.01 min−1). Moreover, MMS with good stability and reusability could reach 92% degradation of SMX after 5 cycles. Quenching experiments and electron spin resonance (ESR) tests revealed that hydroxyl radicals (.OH) and singlet oxygen (1O2) were the dominant reactive oxygen species (ROS) for SMX degradation. The integration of experimental works, characterization techniques and density functional theory (DFT) calculations unraveled that the formation of sulfur vacancies in MMS catalyst could expose more Mo sites, improve the charge density and boost the electron transfer, which was conducive to accelerating the Fe3+/Fe2+ cycle for enhancing the activation of PMS. Finally, the C-N, N-O, S-N, C-O and C-S bonds of SMX were easily attacked by ROS to generate the nontoxic intermediates in the MMS/PMS/SMX system. This study offers a new approach to designing high-performance Fe-MoS2 catalysts for the removal of organic pollutants.
中文翻译:

花环状 MIL-101(Fe)/MoS2 纳米片中 Fe3+/Fe2+ 循环的加速促进过一硫酸盐活化以降解磺胺甲恶唑
铁基二硫化钼(Fe-MoS 2)已成为一种类芬顿催化剂,可用于高效降解抗生素,但其构效关系仍然难以捉摸。在此,制备了具有双金属活性位点(Fe和Mo)和丰富硫空位的花环状MIL-101(Fe)/MoS 2纳米片(MMS),以直接激活过一硫酸盐(PMS),以快速降解不同的有机污染物(酚类) 、染料和药物),甚至在真实的水体中也是如此。MMS在降解磺胺甲恶唑(SMX)时表现出极快的催化速率常数,为0.289 min -1 ,分别是单一MoS 2(0.008 min -1)和MIL-101(Fe)(0.01 min)的36和29倍。 −1)。此外,MMS具有良好的稳定性和可重复使用性,5次循环后SMX降解率达到92%。淬灭实验和电子自旋共振 (ESR) 测试表明,羟基自由基 ( . OH) 和单线态氧 ( 1 O 2 ) 是 SMX 降解的主要活性氧 (ROS)。实验工作、表征技术和密度泛函理论(DFT)计算的结合揭示了MMS催化剂中硫空位的形成可以暴露更多的Mo位点,提高电荷密度并促进电子转移,有利于加速Fe 3 + /Fe 2+循环以增强 PMS 的激活。最后,SMX的CN、NO、SN、CO和CS键很容易被ROS攻击,在MMS/PMS/SMX系统中生成无毒的中间体。这项研究为设计用于去除有机污染物的高性能 Fe-MoS 2催化剂提供了一种新方法。































 京公网安备 11010802027423号
京公网安备 11010802027423号