当前位置:
X-MOL 学术
›
ACS Sustain. Chem. Eng.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Reaction Mechanism and Technical Application of Metallic Bismuth Extraction from Bismuthinite Concentrate by Low-Temperature Alkaline Smelting
ACS Sustainable Chemistry & Engineering ( IF 7.1 ) Pub Date : 2023-06-27 , DOI: 10.1021/acssuschemeng.3c00081 Wei Jin 1 , Shenghai Yang 1, 2 , Chaobo Tang 1, 2 , Yun Li 1 , Cong Chang 1 , Yongming Chen 1, 2
ACS Sustainable Chemistry & Engineering ( IF 7.1 ) Pub Date : 2023-06-27 , DOI: 10.1021/acssuschemeng.3c00081 Wei Jin 1 , Shenghai Yang 1, 2 , Chaobo Tang 1, 2 , Yun Li 1 , Cong Chang 1 , Yongming Chen 1, 2
Affiliation
|
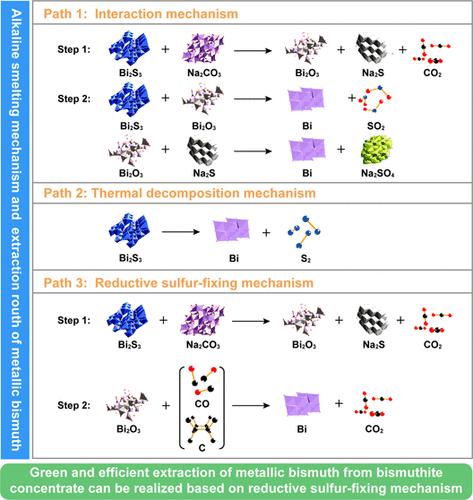
Traditional bismuthinite concentrate precipitation smelting has problems of low recovery of valuable metals (e.g., Bi, Cu, Mo, and Ag), high fuel consumption, and high cost of SO2 flue gas treatment. Therefore, in this study, an eco-friendly alkaline smelting process was proposed to extract metallic bismuth and recover valuable metals. The phase equilibrium composition of the Bi2S3-Na2CO3-C system was revealed by thermodynamic simulations at different temperatures and reductant contents. Based on reaction product characterizations and thermodynamic simulations, the following reaction mechanism and suitable bismuth extraction path were confirmed: Bi2S3 first reacted with Na2CO3 to form Bi2O3 and Na2S, and the intermediate (Bi2O3) was then reduced to metallic Bi by both C and CO. Under optimized conditions (Wbismuthinite/WNa2CO3/Wcoke = 200:300:20, 900 °C, 150 min), up to 96.56% Bi, 75.07% Pb, and 85.11% Ag were enriched in crude bismuth, respectively, while 93.57% Cu, 70.23% Fe, 96.63% Mo, and 94.34% W entered the salt slag. More than 99.9% W and Mo were recovered as Na2WO4 and Na2MoO4, respectively, from the salt slag by water leaching. In the leaching residue, chalcopyrite, pyrite, and elemental sulfur could be recovered by flotation.
中文翻译:

低温碱熔炼铋精矿提取金属铋的反应机理及技术应用
传统的铋精矿沉淀熔炼存在Bi、Cu、Mo、Ag等有价金属回收率低、燃料消耗高、SO 2烟气处理成本高等问题。因此,本研究提出了一种环境友好的碱性冶炼工艺来提取金属铋并回收有价金属。通过不同温度和还原剂含量下的热力学模拟揭示了Bi 2 S 3 -Na 2 CO 3 -C体系的相平衡组成。基于反应产物表征和热力学模拟,确定了以下反应机理和合适的铋提取路径: Bi 2 S 3首先与Na 2 CO 3反应生成Bi 2 O 3和Na 2 S,然后中间体(Bi 2 O 3 )被C和CO还原为金属Bi。在优化条件下( W bismuthinite / W Na2CO3 / W焦炭= 200:300:20, 900 °C, 150 min),粗铋中分别富集了高达96.56% Bi、75.07% Pb和85.11% Ag,而粗铋中富集了93.57% Cu、70.23% Fe、96.63% Mo ,94.34%的W进入盐渣。99.9%以上的W和Mo以Na 2 WO 4和Na 2 MoO 4的形式回收分别由盐渣经水浸出而成。浸出渣中可浮选回收黄铜矿、黄铁矿和元素硫。
更新日期:2023-06-27
中文翻译:

低温碱熔炼铋精矿提取金属铋的反应机理及技术应用
传统的铋精矿沉淀熔炼存在Bi、Cu、Mo、Ag等有价金属回收率低、燃料消耗高、SO 2烟气处理成本高等问题。因此,本研究提出了一种环境友好的碱性冶炼工艺来提取金属铋并回收有价金属。通过不同温度和还原剂含量下的热力学模拟揭示了Bi 2 S 3 -Na 2 CO 3 -C体系的相平衡组成。基于反应产物表征和热力学模拟,确定了以下反应机理和合适的铋提取路径: Bi 2 S 3首先与Na 2 CO 3反应生成Bi 2 O 3和Na 2 S,然后中间体(Bi 2 O 3 )被C和CO还原为金属Bi。在优化条件下( W bismuthinite / W Na2CO3 / W焦炭= 200:300:20, 900 °C, 150 min),粗铋中分别富集了高达96.56% Bi、75.07% Pb和85.11% Ag,而粗铋中富集了93.57% Cu、70.23% Fe、96.63% Mo ,94.34%的W进入盐渣。99.9%以上的W和Mo以Na 2 WO 4和Na 2 MoO 4的形式回收分别由盐渣经水浸出而成。浸出渣中可浮选回收黄铜矿、黄铁矿和元素硫。





















































 京公网安备 11010802027423号
京公网安备 11010802027423号