当前位置:
X-MOL 学术
›
Org. Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Chemo- and Diastereoselective Synthesis of Spirooxindole-pyrazolines and Pyrazolones via P(NMe2)3-Mediated Substrate-Controlled Annulations of Azoalkenes with α-Dicarbonyl Compounds
Organic Letters ( IF 4.9 ) Pub Date : 2023-06-26 , DOI: 10.1021/acs.orglett.3c01348
Yunfeng Du 1 , Yuefei Liu 1 , Hongyu Guo 1 , Rongfang Liu 2 , Rong Zhou 1, 3
Organic Letters ( IF 4.9 ) Pub Date : 2023-06-26 , DOI: 10.1021/acs.orglett.3c01348
Yunfeng Du 1 , Yuefei Liu 1 , Hongyu Guo 1 , Rongfang Liu 2 , Rong Zhou 1, 3
Affiliation
|
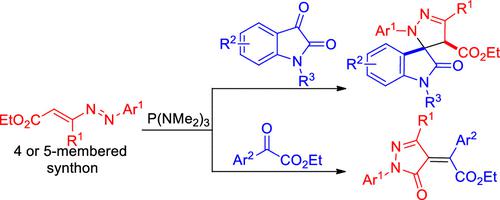
P(NMe2)3-mediated substrate-controlled annulations of azoalkenes with α-dicarbonyl compounds are reported, where the azoalkenes serve as either four or five-atom synthons chemoselectively. The azoalkene participates in annulation with isatins as a four-atom synthon to furnish the spirooxindole-pyrazolines, whereas it functions as a novel five-atom synthon in annulation with aroylformates, thereby leading to chemo- and stereoselective formation of pyrazolones. The synthetic utilities of the annulations have been demonstrated, and a novel TEMPO-mediated decarbonylation reaction is unveiled.
中文翻译:

通过 P(NMe2)3 介导的偶氮烯烃与 α-二羰基化合物的底物控制环化化学和非对映选择性合成螺吲哚吡唑啉和吡唑啉酮
报道了P(NMe 2 ) 3介导的偶氮烯烃与α-二羰基化合物的底物控制环化,其中偶氮烯烃化学选择性地充当四或五原子合成子。偶氮烯烃作为四原子合成子参与与靛红的成环,以提供螺吲哚-吡唑啉,而它在与芳酰甲酸酯的成环中充当新型五原子合成子,从而导致吡唑啉酮的化学和立体选择性形成。环化的合成效用已得到证明,并且揭示了一种新型 TEMPO 介导的脱羰反应。
更新日期:2023-06-26
中文翻译:

通过 P(NMe2)3 介导的偶氮烯烃与 α-二羰基化合物的底物控制环化化学和非对映选择性合成螺吲哚吡唑啉和吡唑啉酮
报道了P(NMe 2 ) 3介导的偶氮烯烃与α-二羰基化合物的底物控制环化,其中偶氮烯烃化学选择性地充当四或五原子合成子。偶氮烯烃作为四原子合成子参与与靛红的成环,以提供螺吲哚-吡唑啉,而它在与芳酰甲酸酯的成环中充当新型五原子合成子,从而导致吡唑啉酮的化学和立体选择性形成。环化的合成效用已得到证明,并且揭示了一种新型 TEMPO 介导的脱羰反应。

































 京公网安备 11010802027423号
京公网安备 11010802027423号