当前位置:
X-MOL 学术
›
ACS Energy Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Acidic Electroreduction of CO2 to Multi-Carbon Products with CO2 Recovery and Recycling from Carbonate
ACS Energy Letters ( IF 19.3 ) Pub Date : 2023-06-26 , DOI: 10.1021/acsenergylett.3c00901 Alessandro Perazio 1 , Charles E. Creissen 2 , José Guillermo Rivera de la Cruz 1 , Moritz W. Schreiber 3 , Marc Fontecave 1
ACS Energy Letters ( IF 19.3 ) Pub Date : 2023-06-26 , DOI: 10.1021/acsenergylett.3c00901 Alessandro Perazio 1 , Charles E. Creissen 2 , José Guillermo Rivera de la Cruz 1 , Moritz W. Schreiber 3 , Marc Fontecave 1
Affiliation
|
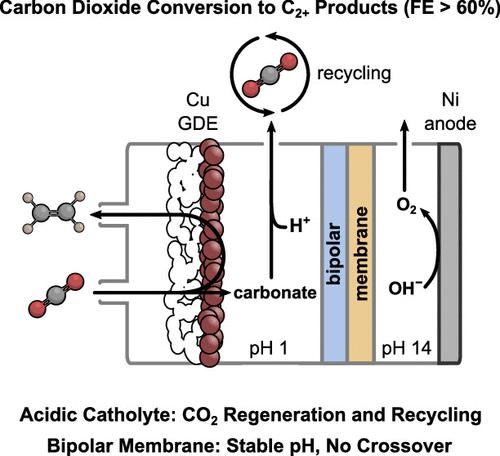
Gas-fed flow cells can facilitate high-rate electrochemical CO2 reduction (CO2R). However, under alkaline and neutral conditions, CO2 is lost through reaction with hydroxide ions to form (bi)carbonate. In acidic solutions, although (bi)carbonate is still formed due to increased pH at the electrode, the low bulk pH of the electrolyte solution can regenerate CO2 which is then available for re-reaction or release─this therefore avoids permanent CO2 loss. Here, we show how CO2 is converted and released in a bipolar-membrane-based gas-fed flow cell for CO2R to multicarbon products (C2+ faradaic efficiency >60%) employing an acidic catholyte. Under the highest conversion conditions, we showed that almost exclusively CO2R products were obtained at one outlet, while, at the second outlet, a nearly product-free stream of CO2 was obtained due to the continuous internal regeneration from (bi)carbonate. The system presented here avoids permanent reactant loss through the straightforward recovery and recycling of CO2 to improve the overall CO2 utilization.
中文翻译:

酸性电还原 CO2 生成多碳产品,并从碳酸盐中回收和再循环 CO2
供气流通池可以促进高速电化学CO 2还原(CO 2 R)。然而,在碱性和中性条件下,CO 2通过与氢氧根离子反应形成碳酸(氢)盐而损失。在酸性溶液中,虽然由于电极 pH 值升高仍会形成碳酸氢盐,但电解质溶液的低本体 pH 值可再生 CO 2,然后可用于重新反应或释放 — 因此可避免永久性 CO 2损失。在这里,我们展示了 CO 2如何在基于双极膜的气体供给流通池中转化和释放,将 CO 2 R 转化为多碳产品 (C 2+使用酸性阴极电解液的法拉第效率 >60%)。在最高转化率条件下,我们发现在一个出口处几乎完全获得了 CO 2 R 产物,而在第二个出口处,由于碳酸氢盐的连续内部再生,获得了几乎无产物的 CO 2流。 。这里介绍的系统通过直接回收和再循环CO 2来避免永久性反应物损失,从而提高CO 2的总体利用率。
更新日期:2023-06-26
中文翻译:

酸性电还原 CO2 生成多碳产品,并从碳酸盐中回收和再循环 CO2
供气流通池可以促进高速电化学CO 2还原(CO 2 R)。然而,在碱性和中性条件下,CO 2通过与氢氧根离子反应形成碳酸(氢)盐而损失。在酸性溶液中,虽然由于电极 pH 值升高仍会形成碳酸氢盐,但电解质溶液的低本体 pH 值可再生 CO 2,然后可用于重新反应或释放 — 因此可避免永久性 CO 2损失。在这里,我们展示了 CO 2如何在基于双极膜的气体供给流通池中转化和释放,将 CO 2 R 转化为多碳产品 (C 2+使用酸性阴极电解液的法拉第效率 >60%)。在最高转化率条件下,我们发现在一个出口处几乎完全获得了 CO 2 R 产物,而在第二个出口处,由于碳酸氢盐的连续内部再生,获得了几乎无产物的 CO 2流。 。这里介绍的系统通过直接回收和再循环CO 2来避免永久性反应物损失,从而提高CO 2的总体利用率。






























 京公网安备 11010802027423号
京公网安备 11010802027423号