当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Fungal P450 Deconstructs the 2,5-Diazabicyclo[2.2.2]octane Ring En Route to the Complete Biosynthesis of 21R-Citrinadin A
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2023-06-23 , DOI: 10.1021/jacs.3c02109 Shuai Liu 1 , Qiuyue Nie 1 , Zhiwen Liu 1 , Siddhant Patil 1 , Xue Gao 1, 2, 3
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2023-06-23 , DOI: 10.1021/jacs.3c02109 Shuai Liu 1 , Qiuyue Nie 1 , Zhiwen Liu 1 , Siddhant Patil 1 , Xue Gao 1, 2, 3
Affiliation
|
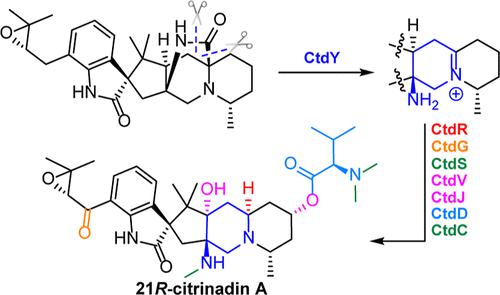
Prenylated indole alkaloids (PIAs) possess great structural diversity and show biological activities. Despite significant efforts in investigating the biosynthetic mechanism, the key step in the transformation of 2,5-diazabicyclo[2.2.2]octane-containing PIAs into a distinct class of pentacyclic compounds remains unknown. Here, using a combination of gene deletion, heterologous expression, and biochemical characterization, we show that a unique fungal P450 enzyme CtdY catalyzes the cleavage of the amide bond in the 2,5-diazabicyclo[2.2.2]octane system, followed by a decarboxylation step to form the 6/5/5/6/6 pentacyclic ring in 21R-citrinadin A. We also demonstrate the function of a subsequent cascade of stereospecific oxygenases to further modify the 6/5/5/6/6 pentacyclic intermediate en route to the complete 21R-citrinadin A biosynthesis. Our findings reveal a key enzyme CtdY for the pathway divergence in the biosynthesis of PIAs and uncover the complex late-stage post-translational modifications in 21R-citrinadin A biosynthesis.
中文翻译:

真菌 P450 在完成 21R-Citrinadin A 生物合成的过程中解构 2,5-二氮杂双环[2.2.2]辛烷环
异戊二烯化吲哚生物碱(PIA)具有巨大的结构多样性并表现出生物活性。尽管在研究生物合成机制方面付出了巨大的努力,但将含有 2,5-二氮杂双环[2.2.2]辛烷的 PIA 转化为一类独特的五环化合物的关键步骤仍然未知。在这里,结合基因删除、异源表达和生化表征,我们证明了一种独特的真菌 P450 酶 CtdY 催化 2,5-二氮杂双环[2.2.2]辛烷系统中酰胺键的裂解,随后脱羧步骤,在 21 R -citrinadin A 中形成 6/5/5/6/6 五环。我们还演示了随后的立体特异性加氧酶级联的功能,以进一步修饰 6/5/5/6/6 五环中间体完成 21 R -citrinadin A 生物合成的途中。我们的研究结果揭示了 PIA 生物合成途径分歧的关键酶 CtdY,并揭示了 21 R -citrinadin A 生物合成中复杂的后期翻译后修饰。
更新日期:2023-06-23
中文翻译:

真菌 P450 在完成 21R-Citrinadin A 生物合成的过程中解构 2,5-二氮杂双环[2.2.2]辛烷环
异戊二烯化吲哚生物碱(PIA)具有巨大的结构多样性并表现出生物活性。尽管在研究生物合成机制方面付出了巨大的努力,但将含有 2,5-二氮杂双环[2.2.2]辛烷的 PIA 转化为一类独特的五环化合物的关键步骤仍然未知。在这里,结合基因删除、异源表达和生化表征,我们证明了一种独特的真菌 P450 酶 CtdY 催化 2,5-二氮杂双环[2.2.2]辛烷系统中酰胺键的裂解,随后脱羧步骤,在 21 R -citrinadin A 中形成 6/5/5/6/6 五环。我们还演示了随后的立体特异性加氧酶级联的功能,以进一步修饰 6/5/5/6/6 五环中间体完成 21 R -citrinadin A 生物合成的途中。我们的研究结果揭示了 PIA 生物合成途径分歧的关键酶 CtdY,并揭示了 21 R -citrinadin A 生物合成中复杂的后期翻译后修饰。






























 京公网安备 11010802027423号
京公网安备 11010802027423号