Acta Neuropathologica ( IF 9.3 ) Pub Date : 2023-06-24 , DOI: 10.1007/s00401-023-02604-x Shelley L Forrest 1, 2, 3 , Seojin Lee 1 , Nasna Nassir 4 , Ivan Martinez-Valbuena 1 , Valerie Sackmann 1 , Jun Li 1 , Awab Ahmed 4 , Maria Carmela Tartaglia 1, 5 , Lars M Ittner 2 , Anthony E Lang 6 , Mohammed Uddin 4, 7 , Gabor G Kovacs 1, 2, 3, 6, 8
|
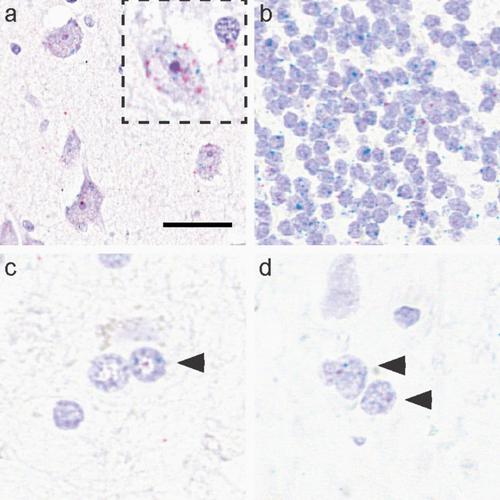
Microtubule-associated protein tau (MAPT) aggregates in neurons, astrocytes and oligodendrocytes in a number of neurodegenerative diseases, including progressive supranuclear palsy (PSP). Tau is a target of therapy and the strategy includes either the elimination of pathological tau aggregates or reducing MAPT expression, and thus the amount of tau protein made to prevent its aggregation. Disease-associated tau affects brain regions in a sequential manner that includes cell-to-cell spreading. Involvement of glial cells that show tau aggregates is interpreted as glial cells taking up misfolded tau assuming that glial cells do not express enough MAPT. Although studies have evaluated MAPT expression in human brain tissue homogenates, it is not clear whether MAPT expression is compromised in cells accumulating pathological tau. To address these perplexing aspects of disease pathogenesis, this study used RNAscope combined with immunofluorescence (AT8), and single-nuclear(sn) RNAseq to systematically map and quantify MAPT expression dynamics across different cell types and brain regions in controls (n = 3) and evaluated whether tau cytopathology affects MAPT expression in PSP (n = 3). MAPT transcripts were detected in neurons, astrocytes and oligodendrocytes, and varied between brain regions and within each cell type, and were preserved in all cell types with tau aggregates in PSP. These results propose a complex scenario in all cell types, where, in addition to the ingested misfolded tau, the preserved cellular MAPT expression provides a pool for local protein production that can (1) be phosphorylated and aggregated, or (2) feed the seeding of ingested misfolded tau by providing physiological tau, both accentuating the pathological process. Since tau cytopathology does not compromise MAPT gene expression in PSP, a complete loss of tau protein expression as an early pathogenic component is less likely. These observations provide rationale for a dual approach to therapy by decreasing cellular MAPT expression and targeting removal of misfolded tau.
中文翻译:

进行性核上性麻痹的神经元和神经胶质 tau 细胞病理学中保留了细胞特异性 MAPT 基因表达
微管相关蛋白 tau ( MAPT ) 在许多神经退行性疾病中的神经元、星形胶质细胞和少突胶质细胞中聚集,包括进行性核上性麻痹 (PSP)。 Tau 是治疗的目标,策略包括消除病理性 tau 聚集物或减少MAPT表达,从而减少 tau 蛋白的量以防止其聚集。与疾病相关的 tau 蛋白以连续的方式影响大脑区域,包括细胞间的传播。显示 tau 聚集的神经胶质细胞的参与被解释为假设神经胶质细胞没有表达足够的MAPT ,则神经胶质细胞吸收了错误折叠的 tau 。尽管研究评估了人脑组织匀浆中的MAPT表达,但尚不清楚累积病理性 tau 细胞中MAPT表达是否受到损害。为了解决疾病发病机制中这些令人困惑的问题,本研究使用 RNAscope 结合免疫荧光 (AT8) 和单核 (sn) RNAseq 来系统地绘制和量化对照中不同细胞类型和大脑区域的MAPT表达动态 ( n = 3)并评估 tau 细胞病理学是否影响 PSP 中的MAPT表达( n = 3)。 MAPT转录本在神经元、星形胶质细胞和少突胶质细胞中检测到,并且在大脑区域之间和每种细胞类型内有所不同,并且在 PSP 中与 tau 聚集体一起保存在所有细胞类型中。 这些结果提出了所有细胞类型中的复杂情况,其中除了摄入的错误折叠 tau 之外,保留的细胞MAPT表达还为局部蛋白质生产提供了一个库,可以(1)被磷酸化和聚集,或(2)为种子提供营养通过提供生理性 tau 蛋白来抑制摄入的错误折叠 tau 蛋白,两者都会加剧病理过程。由于 tau 细胞病理学不会损害 PSP 中的MAPT基因表达,因此作为早期致病成分的 tau 蛋白表达完全丧失的可能性较小。这些观察结果为减少细胞MAPT表达和靶向去除错误折叠 tau 蛋白的双重治疗方法提供了理论基础。































 京公网安备 11010802027423号
京公网安备 11010802027423号