Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Nanofluidic Aptamer Nanoarray to Enable Stochastic Capture of Single Proteins at Normal Concentrations
Small ( IF 13.0 ) Pub Date : 2023-06-23 , DOI: 10.1002/smll.202301013 Jinbin Yang 1 , Hiroki Kamai 1 , Yong Wang 2 , Yan Xu 1, 3, 4, 5
Small ( IF 13.0 ) Pub Date : 2023-06-23 , DOI: 10.1002/smll.202301013 Jinbin Yang 1 , Hiroki Kamai 1 , Yong Wang 2 , Yan Xu 1, 3, 4, 5
Affiliation
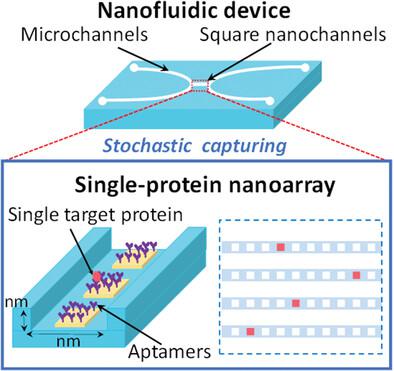
|
Single-molecule experiments allow understanding of the diversity, stochasticity, and heterogeneity of molecular behaviors and properties hidden by conventional ensemble-averaged measurements. They hence have great importance and significant impacts in a wide range of fields. Despite significant advances in single-molecule experiments at ultralow concentrations, the capture of single molecules in solution at normal concentrations within natural biomolecular processes remains a formidable challenge. Here, a high-density, well-defined nanofluidic aptamer nanoarray (NANa) formed via site-specific self-assembly of well-designed aptamer molecules in nanochannels with nano-in-nano gold nanopatterns is presented. The nanofluidic aptamer nanoarray exhibits a high capability to specifically capture target proteins (e.g., platelet-derived growth factor BB; PDGF-BB) to form uniform protein nanoarrays under optimized nanofluidic conditions. Owing to these fundamental features, the nanofluidic aptamer nanoarray enables the stochastic capture of single PDGF-BB molecules at a normal concentration from a sample with an ultrasmall volume equivalent to a single cell by following Poisson statistics, forming a readily addressable single-protein nanoarray. This approach offers a methodology and device to surpass both the concentration and volume limits of single-protein capture in most conventional methodologies of single-molecule experiments, thus opening an avenue to explore the behavior of individual biomolecules in a manner close to their natural forms, which remains largely unexplored to date.
中文翻译:

纳米流体适体纳米阵列能够在正常浓度下随机捕获单一蛋白质
单分子实验可以了解传统整体平均测量所隐藏的分子行为和特性的多样性、随机性和异质性。因此,它们在广泛的领域中具有非常重要的意义和重大影响。尽管超低浓度的单分子实验取得了重大进展,但在自然生物分子过程中捕获正常浓度溶液中的单分子仍然是一个艰巨的挑战。在这里,提出了一种高密度、明确的纳米流体适体纳米阵列(NANa),它是通过精心设计的适体分子在纳米通道中进行位点特异性自组装而形成的,具有纳米级纳米金纳米图案。纳米流体适体纳米阵列表现出特异性捕获靶蛋白(例如血小板衍生生长因子BB;PDGF-BB)的高性能,以在优化的纳米流体条件下形成均匀的蛋白质纳米阵列。由于这些基本特征,纳流适体纳米阵列能够按照泊松统计从相当于单细胞的超小体积样品中随机捕获正常浓度的单个PDGF-BB分子,形成易于寻址的单蛋白纳米阵列。这种方法提供了一种方法和设备,超越了大多数传统单分子实验方法中单蛋白捕获的浓度和体积限制,从而开辟了一条以接近其自然形式的方式探索单个生物分子行为的途径,迄今为止,这在很大程度上仍未被探索。
更新日期:2023-06-23
中文翻译:

纳米流体适体纳米阵列能够在正常浓度下随机捕获单一蛋白质
单分子实验可以了解传统整体平均测量所隐藏的分子行为和特性的多样性、随机性和异质性。因此,它们在广泛的领域中具有非常重要的意义和重大影响。尽管超低浓度的单分子实验取得了重大进展,但在自然生物分子过程中捕获正常浓度溶液中的单分子仍然是一个艰巨的挑战。在这里,提出了一种高密度、明确的纳米流体适体纳米阵列(NANa),它是通过精心设计的适体分子在纳米通道中进行位点特异性自组装而形成的,具有纳米级纳米金纳米图案。纳米流体适体纳米阵列表现出特异性捕获靶蛋白(例如血小板衍生生长因子BB;PDGF-BB)的高性能,以在优化的纳米流体条件下形成均匀的蛋白质纳米阵列。由于这些基本特征,纳流适体纳米阵列能够按照泊松统计从相当于单细胞的超小体积样品中随机捕获正常浓度的单个PDGF-BB分子,形成易于寻址的单蛋白纳米阵列。这种方法提供了一种方法和设备,超越了大多数传统单分子实验方法中单蛋白捕获的浓度和体积限制,从而开辟了一条以接近其自然形式的方式探索单个生物分子行为的途径,迄今为止,这在很大程度上仍未被探索。































 京公网安备 11010802027423号
京公网安备 11010802027423号