当前位置:
X-MOL 学术
›
J. Mater. Chem. C
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
ACQ to AIE transformation of quinoline derivatives: modulating substituent electronic effects to alter excited-state reorganization energy distribution
Journal of Materials Chemistry C ( IF 5.7 ) Pub Date : 2023-06-22 , DOI: 10.1039/d3tc01519j Longjie Wang 1 , Yuchen Zhang 2 , Xiangdi Huang 1 , Yanxiong Liu 1 , Yi Cheng 1 , Wenwen Fan 1 , Liyan Zheng 1 , Qiue Cao 1
Journal of Materials Chemistry C ( IF 5.7 ) Pub Date : 2023-06-22 , DOI: 10.1039/d3tc01519j Longjie Wang 1 , Yuchen Zhang 2 , Xiangdi Huang 1 , Yanxiong Liu 1 , Yi Cheng 1 , Wenwen Fan 1 , Liyan Zheng 1 , Qiue Cao 1
Affiliation
|
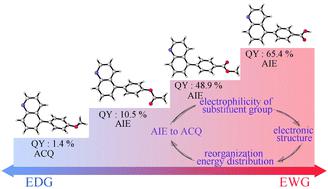
Since the discovery of aggregation-induced emission (AIE), the development of new AIE fluorogens (AIEgens) has become a significant field owing to their versatile application in diverse fields. To achieve aggregation-caused quenching (ACQ) to AIE conversion, considerable attention is paid to the strategy of changing the stacking pattern of molecules, and several researchers are focused on modulating the electronic structure of the excited state of molecules. In this study, acetoxy and methoxy electron-donating groups (EDG) and carboxyl and ester electron-withdrawing groups (EWG) were introduced onto quinoline through a facile Suzuki coupling reaction. The experimental results reveal that the behavior of quinoline derivatives can be converted from ACQ to AIE by altering substituents from EDG to EWG. Single-crystal X-ray diffraction (SCXRD) and transient and steady-state spectra were employed to reveal that their AIE mechanism involved the restriction of intramolecular motion (RIM). In addition, density functional theory (DFT) calculations showed that the ACQ to AIE transition can be achieved by changing the reorganization energy distribution of the molecule with different substituents in the excited state. Furthermore, the coordination of quinoline nitrogen atoms to Fe3+ facilitated the production of larger nanofibers that possessed enhanced AIE properties. This unique characteristic was utilized to leverage the capabilities of methyl 4-(quinolin-5-yl)benzoate (5-MQB) as a ratiometric fluorescent probe for detecting Fe3+ in aqueous solution.
中文翻译:

喹啉衍生物的 ACQ 到 AIE 转化:调节取代基电子效应以改变激发态重组能量分布
自从聚集诱导发射(AIE)被发现以来,新型 AIE 荧光剂(AIEgens)的开发由于其在不同领域的广泛应用而成为一个重要领域。为了实现聚集引起的猝灭(ACQ)到AIE的转换,改变分子堆积模式的策略受到了相当多的关注,一些研究人员致力于调节分子激发态的电子结构。在这项研究中,通过简单的铃木偶联反应将乙酰氧基和甲氧基给电子基团(EDG)以及羧基和酯吸电子基团(EWG)引入到喹啉上。实验结果表明,通过将取代基从EDG改为EWG,可以将喹啉衍生物的行为从ACQ转变为AIE。采用单晶 X 射线衍射 (SCXRD) 以及瞬态和稳态光谱揭示了它们的 AIE 机制涉及分子内运动 (RIM) 的限制。此外,密度泛函理论(DFT)计算表明,通过改变激发态不同取代基分子的重组能分布,可以实现ACQ到AIE的转变。此外,喹啉氮原子与 Fe 的配位3+促进了具有增强 AIE 特性的较大纳米纤维的生产。利用这种独特的特性,利用 4-(喹啉-5-基)苯甲酸甲酯 (5-MQB) 作为比率荧光探针来检测水溶液中的Fe 3+的能力。
更新日期:2023-06-23
中文翻译:

喹啉衍生物的 ACQ 到 AIE 转化:调节取代基电子效应以改变激发态重组能量分布
自从聚集诱导发射(AIE)被发现以来,新型 AIE 荧光剂(AIEgens)的开发由于其在不同领域的广泛应用而成为一个重要领域。为了实现聚集引起的猝灭(ACQ)到AIE的转换,改变分子堆积模式的策略受到了相当多的关注,一些研究人员致力于调节分子激发态的电子结构。在这项研究中,通过简单的铃木偶联反应将乙酰氧基和甲氧基给电子基团(EDG)以及羧基和酯吸电子基团(EWG)引入到喹啉上。实验结果表明,通过将取代基从EDG改为EWG,可以将喹啉衍生物的行为从ACQ转变为AIE。采用单晶 X 射线衍射 (SCXRD) 以及瞬态和稳态光谱揭示了它们的 AIE 机制涉及分子内运动 (RIM) 的限制。此外,密度泛函理论(DFT)计算表明,通过改变激发态不同取代基分子的重组能分布,可以实现ACQ到AIE的转变。此外,喹啉氮原子与 Fe 的配位3+促进了具有增强 AIE 特性的较大纳米纤维的生产。利用这种独特的特性,利用 4-(喹啉-5-基)苯甲酸甲酯 (5-MQB) 作为比率荧光探针来检测水溶液中的Fe 3+的能力。































 京公网安备 11010802027423号
京公网安备 11010802027423号