Nano Research ( IF 9.5 ) Pub Date : 2023-06-21 , DOI: 10.1007/s12274-023-5862-0
Tongxin Song , Jie Kong , Shisi Tang , Xiao Cai , Xu Liu , Meng Zhou , Wen Wu Xu , Weiping Ding , Yan Zhu
|
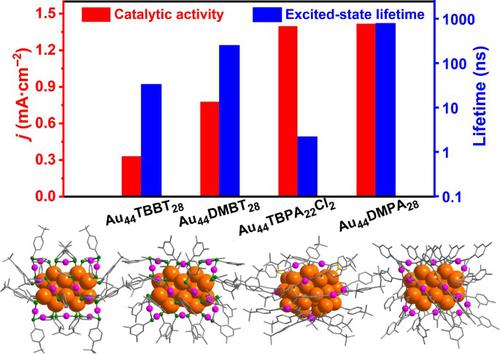
The structure determination of metal nanoclusters protected by ligands is critical in understanding their physical and chemical properties, yet it remains elusive how the metal core and ligand of metal clusters cooperatively contribute to the observed performances. Here, with the successful synthesis of Au44TBPA22Cl2 cluster (TBPA = 4-tert-butylphenylacetylene), the structural isomer of previously reported Au44L28 clusters (L denoted as ligand) is filled, thereby providing an opportunity to explore the property evolution rules imparted by different metal core structures or different surface ligands. Time-resolved transient absorption spectroscopy reveals that the difference in the core structure between Au44TBPA22Cl2 and Au44L28 can bring nearly 360 times variation of excited-state lifetime, while only 3–24 times differences in excited-state lifetimes of the three Au44L28 nanoclusters with identical metal core but different ligands are observed, which is due to much stronger impact of the metal core than the surface ligands in the electronic energy bands of the clusters. In addition, the Au44 clusters protected by alkyne ligands are shown to be highly effective toward the electrochemical oxidation of ethanol, compared to the Au44 clusters capped by thiolates, which is ascribed to smaller charge transfer impedance of the former clusters. We anticipate that the study will enhance the process in controlling the nanomaterial properties by precisely tailoring metal core or surface patterns.
中文翻译:

异构 Au44 团簇对激发态动力学和催化活性的修饰
受配体保护的金属纳米团簇的结构确定对于理解其物理和化学性质至关重要,但金属团簇的金属核和配体如何共同促进所观察到的性能仍然难以捉摸。这里,随着Au 44 TBPA 22 Cl 2团簇(TBPA=4-叔丁基苯乙炔)的成功合成,填补了之前报道的Au 44 L 28团簇(L表示配体)的结构异构体,从而为探索提供了机会。不同金属核心结构或不同表面配体赋予的性能演化规则。时间分辨瞬态吸收光谱揭示了Au和Au之间核心结构的差异44 TBPA 22 Cl 2和 Au 44 L 28可以带来近 360 倍的激发态寿命变化,而具有相同金属核但不同配体的3 个 Au 44 L 28纳米团簇的激发态寿命仅相差 3~24 倍。观察到,这是由于金属核在团簇电子能带中的影响比表面配体强得多。此外,与 Au 44簇相比,受炔配体保护的 Au 44簇对乙醇的电化学氧化非常有效。簇被硫醇盐覆盖,这归因于前簇的电荷转移阻抗较小。我们预计这项研究将通过精确定制金属芯或表面图案来增强控制纳米材料特性的过程。

































 京公网安备 11010802027423号
京公网安备 11010802027423号