当前位置:
X-MOL 学术
›
Inorg. Chem. Front.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
CX-5461-inspired monofunctional platinum RNA polymerase I selective inhibitors with selective lethality in BRCA1-deficient cancer cells
Inorganic Chemistry Frontiers ( IF 6.1 ) Pub Date : 2023-06-20 , DOI: 10.1039/d3qi00358b Zhen-Lei Zhang 1 , Rui Rong 1 , Xuan-Lin Ren 1 , Ling-Wen Xu 1 , Wen-Jing Lian 1 , Xin Qiao 1 , Jing-Yuan Xu 1, 2
Inorganic Chemistry Frontiers ( IF 6.1 ) Pub Date : 2023-06-20 , DOI: 10.1039/d3qi00358b Zhen-Lei Zhang 1 , Rui Rong 1 , Xuan-Lin Ren 1 , Ling-Wen Xu 1 , Wen-Jing Lian 1 , Xin Qiao 1 , Jing-Yuan Xu 1, 2
Affiliation
|
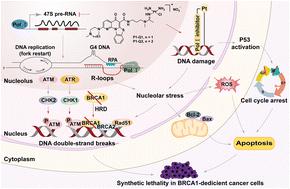
Unrestricted cell growth and proliferation of cancer cells correlate with accelerated hyperactivated RNA polymerase I transcription and upregulation of ribosome biogenesis. Herein, we employed 2-(4-methyl-[1,4]diazepan-1-yl)-5-oxo-5H-7-thia-1,11b-diaza-benzo[c]fluorene-6-carboxylic acid (5-methyl-pyrazin-2-ylmethyl)-amide (CX-5461), the first Pol I selective inhibitor with the multitargeting property and synthetical lethality in homologous recombination (HR) deficient cancers, to modify cisplatin, affording two monofunctional platinum Pol I selective inhibitors [PtCl(NH3)2(LH1)]NO3 (P1-Q1) and [PtCl(NH3)2(LH2)]NO3 (P1-Q2) [LH1 = N-(3-(N-methylacetimidamido)propyl)-2-(4-methylpiperazin-1-yl)-5-oxo-5H-benzo[4,5]thiazolo[3,2-a][1,8]naphthyridine-6-carboxamide; LH2 = 2-(4-methyl-1,4-diazepan-1-yl)-N-(3-(N-methylacetimidamido)propyl)-5-oxo-5H-benzo[4,5]thiazolo[3,2-a][1,8]naphthyridine-6-carboxamide]. Our data showed that P1-Q1 and P1-Q2 could preferentially target the Pol I transcription machinery, overcome platinum drug resistance, and display more potent antiproliferative activity in BRCA1-deficient A549 cells. Mechanism studies demonstrated that P1-Q1 and P1-Q2 could effectively accumulate in cancer cells and nucleoli, selectively inhibit Pol I transcription, stimulate nucleolar stress and p53 stabilization, increase intracellular ROS levels, disrupt the mitochondrial membrane potential, and inhibit migration and metastasis, which further lead to cell cycle arrest and apoptosis. Particularly, P1-Q1 could facilitate the formation and stabilization of R-loops, induce severe DNA damage, and trigger ATR/CHK1 and ATM/CHK2 kinase signaling cascades in BRCA1-deficient A549 cells, suggesting that defects in the HR pathway may exacerbate P1-Q1 induced DNA damage and cause synthetic lethality and apoptosis. Our data demonstrated that P1-Q1 and P1-Q2 are distinct from the clinical platinum anticancer drugs in their mechanism of action, and taking advantage of the selective Pol I inhibition, multitargeting property, and synthetic lethality of CX-5461 in HR-deficient cancers to modify platinum-based agents might be a brand-new approach for cancer therapy.
中文翻译:

CX-5461 启发的单功能铂 RNA 聚合酶 I 选择性抑制剂,对 BRCA1 缺陷癌细胞具有选择性致死作用
癌细胞不受限制的细胞生长和增殖与加速过度激活的 RNA 聚合酶 I 转录和核糖体生物合成的上调相关。在此,我们采用2-(4-甲基-[1,4]二氮杂-1-基)-5-氧代-5 H -7-硫杂-1,11 b-二氮杂-苯并[ c ]芴-6-羧基酸 (5-甲基-吡嗪-2-基甲基)-酰胺 (CX-5461) 是第一个 Pol I 选择性抑制剂,在同源重组 (HR) 缺陷型癌症中具有多靶点特性和合成致死性,可修饰顺铂,提供两个单功能铂Pol I 选择性抑制剂 [PtCl(NH 3 ) 2 ( LH1 )]NO 3 ( P1-Q1 ) 和 [PtCl(NH 3 )2 ( LH2 )]NO 3 ( P1-Q2 ) [ LH1 = N -(3-( N-甲基乙酰脒基)丙基)-2-(4-甲基哌嗪-1-基)-5-氧代-5 H-苯并[4 ,5]噻唑并[3,2- a ][1,8]萘啶-6-甲酰胺;LH2 = 2-(4-甲基-1,4-二氮杂环-1-基)-N- ( 3-( N-甲基乙脒基)丙基)-5-氧代-5 H-苯并[4,5]噻唑并[3, 2- a ][1,8]萘啶-6-甲酰胺]。我们的数据显示P1-Q1和P1-Q2可以优先靶向 Pol I 转录机制,克服铂类药物耐药性,并在 BRCA1 缺陷的 A549 细胞中表现出更有效的抗增殖活性。机制研究表明,P1-Q1和P1-Q2可以有效积聚在癌细胞和核仁中,选择性抑制Pol I转录,刺激核仁应激和p53稳定,增加细胞内ROS水平,破坏线粒体膜电位,抑制迁移和转移。进一步导致细胞周期停滞和细胞凋亡。特别是,P1-Q1可以促进 R 环的形成和稳定,诱导严重的 DNA 损伤,并在 BRCA1 缺陷的 A549 细胞中触发 ATR/CHK1 和 ATM/CHK2 激酶信号级联反应,表明 HR 通路的缺陷可能会加剧 P1-Q1 诱导的DNA损伤并引起综合致死和细胞凋亡。我们的数据表明,P1-Q1和P1-Q2 的作用机制与临床铂类抗癌药物不同,并利用了 CX-5461 在 HR 缺陷癌症中的选择性 Pol I 抑制、多靶点特性和合成致死性改良铂类药物可能是一种全新的癌症治疗方法。
更新日期:2023-06-21
中文翻译:

CX-5461 启发的单功能铂 RNA 聚合酶 I 选择性抑制剂,对 BRCA1 缺陷癌细胞具有选择性致死作用
癌细胞不受限制的细胞生长和增殖与加速过度激活的 RNA 聚合酶 I 转录和核糖体生物合成的上调相关。在此,我们采用2-(4-甲基-[1,4]二氮杂-1-基)-5-氧代-5 H -7-硫杂-1,11 b-二氮杂-苯并[ c ]芴-6-羧基酸 (5-甲基-吡嗪-2-基甲基)-酰胺 (CX-5461) 是第一个 Pol I 选择性抑制剂,在同源重组 (HR) 缺陷型癌症中具有多靶点特性和合成致死性,可修饰顺铂,提供两个单功能铂Pol I 选择性抑制剂 [PtCl(NH 3 ) 2 ( LH1 )]NO 3 ( P1-Q1 ) 和 [PtCl(NH 3 )2 ( LH2 )]NO 3 ( P1-Q2 ) [ LH1 = N -(3-( N-甲基乙酰脒基)丙基)-2-(4-甲基哌嗪-1-基)-5-氧代-5 H-苯并[4 ,5]噻唑并[3,2- a ][1,8]萘啶-6-甲酰胺;LH2 = 2-(4-甲基-1,4-二氮杂环-1-基)-N- ( 3-( N-甲基乙脒基)丙基)-5-氧代-5 H-苯并[4,5]噻唑并[3, 2- a ][1,8]萘啶-6-甲酰胺]。我们的数据显示P1-Q1和P1-Q2可以优先靶向 Pol I 转录机制,克服铂类药物耐药性,并在 BRCA1 缺陷的 A549 细胞中表现出更有效的抗增殖活性。机制研究表明,P1-Q1和P1-Q2可以有效积聚在癌细胞和核仁中,选择性抑制Pol I转录,刺激核仁应激和p53稳定,增加细胞内ROS水平,破坏线粒体膜电位,抑制迁移和转移。进一步导致细胞周期停滞和细胞凋亡。特别是,P1-Q1可以促进 R 环的形成和稳定,诱导严重的 DNA 损伤,并在 BRCA1 缺陷的 A549 细胞中触发 ATR/CHK1 和 ATM/CHK2 激酶信号级联反应,表明 HR 通路的缺陷可能会加剧 P1-Q1 诱导的DNA损伤并引起综合致死和细胞凋亡。我们的数据表明,P1-Q1和P1-Q2 的作用机制与临床铂类抗癌药物不同,并利用了 CX-5461 在 HR 缺陷癌症中的选择性 Pol I 抑制、多靶点特性和合成致死性改良铂类药物可能是一种全新的癌症治疗方法。








































 京公网安备 11010802027423号
京公网安备 11010802027423号