Separation and Purification Technology ( IF 8.1 ) Pub Date : 2023-06-15 , DOI: 10.1016/j.seppur.2023.124345 Zefeng Jiang , Wenqiang Wang , Wenjuan Xue , Hejin Zhu , Mingze Zheng , Hongliang Huang , Chongli Zhong
|
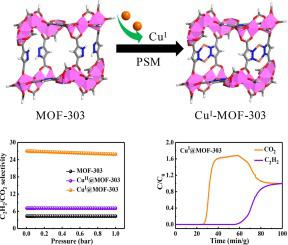
The separation of C2H2/CO2 is very important but still a great challenge in industry because of their very similar molecular sizes and physical properties. Considering that CuI site can form both π complex interactions and π back donation interactions with C2H2, herein, we achieved high C2H2 packing density and efficient C2H2/CO2 separation by anchoring CuI ions with synergistic dual-pyrazol sites of MOF-303. The adsorption experiments show that the resulting CuI@MOF-303 could quickly adsorb C2H2 in the low-pressure region, and the C2H2 packing density is up to 477.9 g/L at 1 bar and 298 K, which is higher than any reported adsorbents. The density functional theory calculations show that π back donation of CuI site in CuI@MOF-303 can strengthen the interaction between CuI@MOF-303 and C2H2 by providing electrons from d orbitals of CuI site to the unoccupied antibonding π* orbital of C2H2 molecule. In addition, the C2H2/CO2 selectivity of CuI@MOF-303 at 1 bar is 25.9, which is 6.0 times of the original MOF-303. Breakthrough experiments show that CuI@MOF-303 has good separation performance for C2H2/CO2 gas mixtures, featuring both high C2H2 uptake and high separation factor of 10.6. Satisfyingly, fuel-grade C2H2 (purity > 98.2 %, 82.83 cm3/cm3) can be obtained after desorption. Moreover, CuI@MOF-303 also has good C2H2 adsorption cycle stability and C2H2/CO2 separation regeneration ability, which indicates that it has great potential in industrial C2H2 purification.
中文翻译:

金属有机框架中π回供活性位点的构建用于C2H2的致密堆积和C2H2/CO2的高效分离
C 2 H 2 /CO 2的分离非常重要,但由于它们的分子大小和物理性质非常相似,因此在工业中仍然是一个巨大的挑战。考虑到Cu I位点可以与C 2 H 2形成π配合物相互作用和π返给相互作用,本文中,我们通过协同锚定Cu I离子实现了高C 2 H 2堆积密度和有效的C 2 H 2 /CO 2分离。 MOF-303 的双吡唑位点。吸附实验表明,所得的Cu I @MOF-303可以快速吸附C2 H 2在低压区,C 2 H 2堆积密度在1 bar和298 K下高达477.9 g/L,高于任何报道的吸附剂。密度泛函理论计算表明,Cu I @MOF-303中Cu I位点的π回馈可以通过将电子从Cu I位点的d轨道提供给空位来增强Cu I @MOF-303和C 2 H 2之间的相互作用。 C 2 H 2分子的反键π*轨道。此外,Cu I的C 2 H 2 /CO 2选择性@MOF-303 在 1 bar 时为 25.9,是原始 MOF-303 的 6.0 倍。突破性的实验表明Cu I @MOF-303对C 2 H 2 /CO 2气体混合物具有良好的分离性能,具有高C 2 H 2吸收率和10.6的高分离因子。解吸后得到了令人满意的燃料级C 2 H 2 (纯度>98.2%,82.83cm 3 /cm 3 )。此外,Cu I @MOF-303还具有良好的C 2 H 2吸附循环稳定性和C 2 H 2 /CO 2分离再生能力强,表明其在工业C 2 H 2纯化方面具有巨大潜力。

































 京公网安备 11010802027423号
京公网安备 11010802027423号