Applied Catalysis B: Environment and Energy ( IF 20.2 ) Pub Date : 2023-06-16 , DOI: 10.1016/j.apcatb.2023.123008 Shuqin Liang , Huashuai Hu , Jue Liu , Hangjia Shen , Qiao Li , Nianxiang Qiu , HaiChuan Guo , Xuyun Guo , Shiyu Du , Ye Zhu , Jian Liu , J. Paul Attfield , Minghui Yang
|
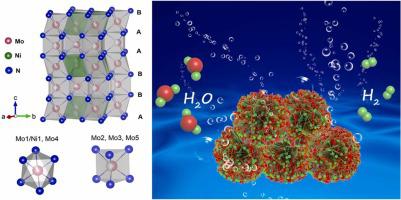
Water electrolysis using the hydrogen evolution reaction (HER) is a promising method for sustainable hydrogen production. Platinum-based catalysts have traditionally been the most efficient HER catalysts, but their scarcity and sluggish water dissociation limit their practical applications. Here we report on a novel superhydrophilic catalyst of nickel supported on nickel molybdenum nitride (Ni/Ni0.8Mo4.2N6) that outperforms platinum-based nanomaterials. Despite the low catalytic activity of Ni or Ni0.8Mo4.2N6 alone, their optimized composite exhibits exceptional HER activity, with respective 500% and 150% increases in the exchange current and turnover frequency compared to commercial Pt/C. Density of states calculations reveal a decrease in electron density of the supported nickel in Ni/Ni0.8Mo4.2N6, leading to a lower free energy of the HER. These findings demonstrate a powerful electron-engineering strategy for designing supported electrocatalysts with outstanding performance for the HER and related processes.
中文翻译:

氮化镍复合材料:用于电催化制氢的铂的环保高效替代品
使用析氢反应(HER)的水电解是一种有前途的可持续制氢方法。铂基催化剂传统上是最有效的HER催化剂,但其稀缺性和缓慢的水解离限制了其实际应用。在这里,我们报道了一种新型镍钼氮化物负载的超亲水催化剂(Ni/Ni 0.8 Mo 4.2 N 6),其性能优于铂基纳米材料。尽管Ni或Ni 0.8 Mo 4.2 N 6的催化活性较低仅凭这一点,他们的优化复合材料就表现出卓越的 HER 活性,与商业 Pt/C 相比,交换电流和周转频率分别增加了 500% 和 150%。态密度计算表明Ni/Ni 0.8 Mo 4.2 N 6中负载镍的电子密度降低,导致HER自由能降低。这些发现证明了一种强大的电子工程策略,可用于设计在 HER 及相关过程中具有出色性能的支撑电催化剂。































 京公网安备 11010802027423号
京公网安备 11010802027423号