Nano Research ( IF 9.5 ) Pub Date : 2023-06-17 , DOI: 10.1007/s12274-023-5791-y Sha Wang , Jianling Zhang , Lei Yao , Yisen Yang , Lirong Zheng , Bo Guan , Yingzhe Zhao , Yanyue Wang , Buxing Han , Xueqing Xing
|
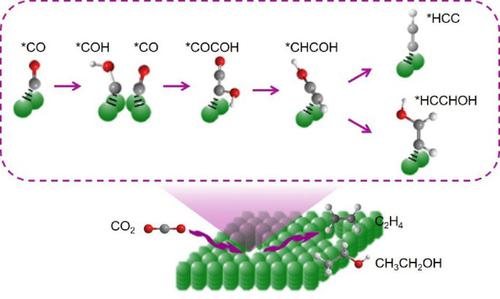
To improve the electrocatalytic conversion of carbon dioxide (CO2) into C2+ products (such as ethylene (C2H4) and ethanol (CH3CH2OH), etc.) is of great importance, but remains challenging. Herein, we proposed a strategy that directs the C-C coupling pathway through enriching and confining the carbon monoxide (CO) intermediate to internal pores of Cu nanocubes, for electrocatalytic reduction of CO2 into C2+ chemicals. In H-type cell, the Faraday efficiency (FE) for ethylene and ethanol reaches 70.3% at −1.28 V versus the reversible hydrogen electrode (vs. RHE), with a current density of 47.9 mA·cm−2. In flow cell, the total current density is up to 340.3 mA·cm−2 at −2.38 V (vs. RHE) and the FE for C2+ products is 67.4%. Experimental and theoretical studies reveal that both the CO intermediate adsorption and C-C coupling reaction on such an internal porous catalyst are facilitated, thus improving CO2-to-C2+ conversion efficiency.
中文翻译:

内部多孔铜上高效电催化 CO2 还原为 C2+ 化学品
提高二氧化碳(CO 2)电催化转化为C 2+产物(如乙烯(C 2 H 4)和乙醇(CH 3 CH 2 OH)等)非常重要,但仍然具有挑战性。在此,我们提出了一种策略,通过将一氧化碳(CO)中间体富集并限制在Cu纳米立方体的内部孔中来引导CC偶联途径,以将CO 2 电催化还原为C 2+化学品。在H型电池中,相对于可逆氢电极(vs. RHE),乙烯和乙醇的法拉第效率(FE)在-1.28 V时达到70.3%,电流密度为47.9 mA·cm -2。在流通池中,在-2.38 V(相对于RHE)下总电流密度高达340.3 mA·cm -2并且C 2+产物的FE为67.4%。实验和理论研究表明,这种内部多孔催化剂上的CO中间体吸附和CC偶联反应均得到促进,从而提高了CO 2 -到C 2+的转化效率。































 京公网安备 11010802027423号
京公网安备 11010802027423号