当前位置:
X-MOL 学术
›
Adv. Funct. Mater.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Impact of Fluorine-Based Lithium Salts on SEI for All-Solid-State PEO-Based Lithium Metal Batteries
Advanced Functional Materials ( IF 18.5 ) Pub Date : 2023-06-13 , DOI: 10.1002/adfm.202303718
Jiajia Li 1, 2 , Haiman Hu 1 , Wenhao Fang 2 , Junwei Ding 1 , Du Yuan 3 , Shuangjiang Luo 2 , Haitao Zhang 2 , Xiaoyan Ji 1
Advanced Functional Materials ( IF 18.5 ) Pub Date : 2023-06-13 , DOI: 10.1002/adfm.202303718
Jiajia Li 1, 2 , Haiman Hu 1 , Wenhao Fang 2 , Junwei Ding 1 , Du Yuan 3 , Shuangjiang Luo 2 , Haitao Zhang 2 , Xiaoyan Ji 1
Affiliation
|
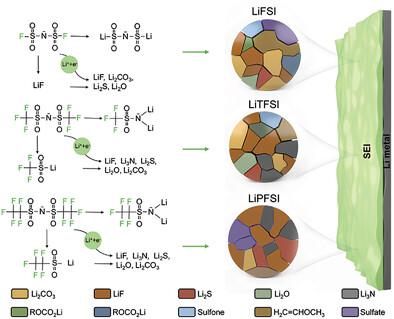
LiF-rich solid-electrolyte-interphase (SEI) can suppress the formation of lithium dendrites and promote the reversible operation of lithium metal batteries. Regulating the composition of naturally formed SEI is an effective strategy, while understanding the impact and role of fluorine (F)-based Li-salts on the SEI characteristics is unavailable. Herein, LiFSI, LiTFSI, and LiPFSI are selected to prepare solid polymer electrolytes (SPEs) with poly(ethylene oxide) and polyimide, investigating the effects of molecular size, F contents and chemical structures (F-connecting bonds) of Li-salts and revealing the formation of LiF in the SEI. It is shown that the F-connecting bond is more significant than the molecular size and F element contents, and thus the performances of cells using LiPFSI are slightly better than LiTFSI and much better than LiFSI. The SPE containing LiPFSI can generate a high amount of LiF, and SPEs containing LiPFSI and LiTFSI can generate Li3N, while there is no Li3N production in the SEI for the SPE containing LiFSI. The preferential breakage bonds in LiPFSI are related to its position to Li anode, where Li-metal as the anode is important in forming LiF, and consequently the LiPFSI reduction mechanism is proposed. This study will boost other energy storage systems beyond Li-ion chemistries.
中文翻译:

氟基锂盐对全固态 PEO 基锂金属电池 SEI 的影响
富含LiF的固体电解质中间相(SEI)可以抑制锂枝晶的形成,促进锂金属电池的可逆运行。调节自然形成的 SEI 的组成是一种有效的策略,但无法了解氟 (F) 基锂盐对 SEI 特性的影响和作用。本文选择LiFSI、LiTFSI和LiPFSI与聚环氧乙烷和聚酰亚胺制备固体聚合物电解质(SPE),研究了Li盐和Li盐的分子尺寸、F含量和化学结构(F连接键)的影响。揭示了 SEI 中 LiF 的形成。结果表明,F连接键比分子尺寸和F元素含量更重要,因此使用LiPFSI的电池的性能略好于LiTFSI,远好于LiFSI。3 N,而对于含有LiFSI的SPE,SEI中没有Li 3 N产生。LiPFSI中的优先断裂键与其在Li阳极中的位置有关,其中Li金属作为阳极对于形成LiF很重要,因此提出了LiPFSI还原机制。这项研究将推动锂离子电池以外的其他储能系统。
更新日期:2023-06-13
中文翻译:

氟基锂盐对全固态 PEO 基锂金属电池 SEI 的影响
富含LiF的固体电解质中间相(SEI)可以抑制锂枝晶的形成,促进锂金属电池的可逆运行。调节自然形成的 SEI 的组成是一种有效的策略,但无法了解氟 (F) 基锂盐对 SEI 特性的影响和作用。本文选择LiFSI、LiTFSI和LiPFSI与聚环氧乙烷和聚酰亚胺制备固体聚合物电解质(SPE),研究了Li盐和Li盐的分子尺寸、F含量和化学结构(F连接键)的影响。揭示了 SEI 中 LiF 的形成。结果表明,F连接键比分子尺寸和F元素含量更重要,因此使用LiPFSI的电池的性能略好于LiTFSI,远好于LiFSI。3 N,而对于含有LiFSI的SPE,SEI中没有Li 3 N产生。LiPFSI中的优先断裂键与其在Li阳极中的位置有关,其中Li金属作为阳极对于形成LiF很重要,因此提出了LiPFSI还原机制。这项研究将推动锂离子电池以外的其他储能系统。







































 京公网安备 11010802027423号
京公网安备 11010802027423号