当前位置:
X-MOL 学术
›
J. Pharmacol. Exp. Ther.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Levetiracetam Pharmacokinetics and Brain Uptake in a Lateral Fluid Percussion Injury Rat Model
Journal of Pharmacology and Experimental Therapeutics ( IF 3.1 ) Pub Date : 2023-08-01 , DOI: 10.1124/jpet.122.001377
Lisa D Coles 1 , Patricia G Saletti 2 , Christos Panagiotis Lisgaras 2 , Pablo M Casillas-Espinosa 2 , Wei Liu 2 , Qianyun Li 2 , Nigel C Jones 2 , Sandy Shultz 2 , Idrish Ali 2 , Rhys Brady 2 , Glenn Yamakawa 2 , Matt Hudson 2 , Juliana Silva 2 , Emma Braine 2 , Usha Mishra 2 , James C Cloyd 2 , Terence J O'Brien 2 , Solomon L Moshé 2 , Aristea S Galanopoulou 2 ,
Journal of Pharmacology and Experimental Therapeutics ( IF 3.1 ) Pub Date : 2023-08-01 , DOI: 10.1124/jpet.122.001377
Lisa D Coles 1 , Patricia G Saletti 2 , Christos Panagiotis Lisgaras 2 , Pablo M Casillas-Espinosa 2 , Wei Liu 2 , Qianyun Li 2 , Nigel C Jones 2 , Sandy Shultz 2 , Idrish Ali 2 , Rhys Brady 2 , Glenn Yamakawa 2 , Matt Hudson 2 , Juliana Silva 2 , Emma Braine 2 , Usha Mishra 2 , James C Cloyd 2 , Terence J O'Brien 2 , Solomon L Moshé 2 , Aristea S Galanopoulou 2 ,
Affiliation
|
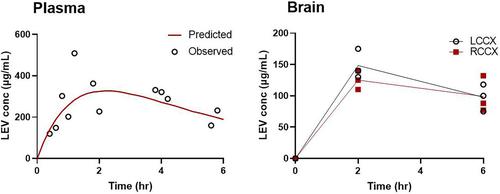
Post-traumatic epilepsy (PTE) occurs in some patients after moderate/severe traumatic brain injury (TBI). Although there are no approved therapies to prevent epileptogenesis, levetiracetam (LEV) is commonly given for seizure prophylaxis due to its good safety profile. This led us to study LEV as part of the Epilepsy Bioinformatics Study for Antiepileptogenic Therapy (EpiBioS4Rx) Project. The objective of this work is to characterize the pharmacokinetics (PK) and brain uptake of LEV in naïve control rats and in the lateral fluid percussion injury (LFPI) rat model of TBI after either single intraperitoneal doses or a loading dose followed by a 7-day subcutaneous infusion. Sprague-Dawley rats were used as controls and for the LFPI model induced at the left parietal region using injury parameters optimized for moderate/severe TBI. Naïve and LFPI rats received either a bolus injection (intraperitoneal) or a bolus injection followed by subcutaneous infusion over 7 days. Blood and parietal cortical samples were collected at specified time points throughout the study. LEV concentrations in plasma and brain were measured using validated high-performance liquid chromatography–tandem mass spectrometry (HPLC-MS/MS) methods. Noncompartmental analysis and a naive-pooled compartmental PK modeling approach were used. Brain-to-plasma ratios ranged from 0.54 to 1.4 to 1. LEV concentrations were well fit by one-compartment, first-order absorption PK models with a clearance of 112 ml/h per kg and volume of distribution of 293 ml/kg. The single-dose pharmacokinetic data were used to guide dose selection for the longer-term studies, and target drug exposures were confirmed. Obtaining LEV PK information early in the screening phase allowed us to guide optimal treatment protocols in EpiBioS4Rx.
中文翻译:

左乙拉西坦在侧向液叩击损伤大鼠模型中的药代动力学和脑摄取
创伤后癫痫 (PTE) 发生在中度/重度创伤性脑损伤 (TBI) 后的一些患者中。虽然没有批准的预防癫痫发生的疗法,但左乙拉西坦 (LEV) 由于其良好的安全性,通常用于预防癫痫发作。这导致我们研究 LEV 作为抗癫痫原治疗生物信息学研究 (EpiBioS4Rx) 项目的一部分。这项工作的目的是表征幼稚对照大鼠和外侧液体叩击损伤 (LFPI) 大鼠模型中 LEV 的药代动力学 (PK) 和脑摄取 TBI 在单次腹膜内给药或负荷剂量后皮下输注 7 天后。Sprague-Dawley 大鼠用作对照,并使用针对中度/重度 TBI 优化的损伤参数在左顶叶区域诱导 LFPI 模型。幼稚大鼠和 LFPI 大鼠接受推注 (腹膜内) 或推注,然后在 7 天内皮下输注。在整个研究的指定时间点收集血液和壁叶皮质样本。使用经过验证的高效液相色谱-串联质谱 (HPLC-MS/MS) 方法测量血浆和脑中的 LEV 浓度。使用非房室分析和朴素混合房室 PK 建模方法。脑浆比值范围为 0.54 至 1.4:1。LEV 浓度与单室、一级吸收 PK 模型拟合良好,清除率为 112 ml/h/kg,分布容积为 293 ml/kg。单剂量药代动力学数据用于指导长期研究的剂量选择,并确认了靶点药物暴露。在筛选阶段的早期获得 LEV PK 信息使我们能够指导 EpiBioS4Rx 中的最佳治疗方案。
更新日期:2023-07-17
中文翻译:

左乙拉西坦在侧向液叩击损伤大鼠模型中的药代动力学和脑摄取
创伤后癫痫 (PTE) 发生在中度/重度创伤性脑损伤 (TBI) 后的一些患者中。虽然没有批准的预防癫痫发生的疗法,但左乙拉西坦 (LEV) 由于其良好的安全性,通常用于预防癫痫发作。这导致我们研究 LEV 作为抗癫痫原治疗生物信息学研究 (EpiBioS4Rx) 项目的一部分。这项工作的目的是表征幼稚对照大鼠和外侧液体叩击损伤 (LFPI) 大鼠模型中 LEV 的药代动力学 (PK) 和脑摄取 TBI 在单次腹膜内给药或负荷剂量后皮下输注 7 天后。Sprague-Dawley 大鼠用作对照,并使用针对中度/重度 TBI 优化的损伤参数在左顶叶区域诱导 LFPI 模型。幼稚大鼠和 LFPI 大鼠接受推注 (腹膜内) 或推注,然后在 7 天内皮下输注。在整个研究的指定时间点收集血液和壁叶皮质样本。使用经过验证的高效液相色谱-串联质谱 (HPLC-MS/MS) 方法测量血浆和脑中的 LEV 浓度。使用非房室分析和朴素混合房室 PK 建模方法。脑浆比值范围为 0.54 至 1.4:1。LEV 浓度与单室、一级吸收 PK 模型拟合良好,清除率为 112 ml/h/kg,分布容积为 293 ml/kg。单剂量药代动力学数据用于指导长期研究的剂量选择,并确认了靶点药物暴露。在筛选阶段的早期获得 LEV PK 信息使我们能够指导 EpiBioS4Rx 中的最佳治疗方案。

































 京公网安备 11010802027423号
京公网安备 11010802027423号