Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Kinetics of hydrogen adsorption and mobility on Ru nanoparticles supported on alumina: Effects on the catalytic mechanism of ammonia synthesis
Journal of Catalysis ( IF 6.5 ) Pub Date : 2016-09-29 17:08:50 Camila Fernández, Nicolas Bion, Eric M. Gaigneaux, Daniel Duprez, Patricio Ruiz
Journal of Catalysis ( IF 6.5 ) Pub Date : 2016-09-29 17:08:50 Camila Fernández, Nicolas Bion, Eric M. Gaigneaux, Daniel Duprez, Patricio Ruiz
|
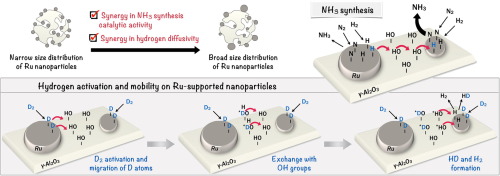
Relevant findings on hydrogen adsorption and mobility are provided in this work to elucidate the mechanism of low-temperature ammonia synthesis, catalyzed by polydispersed Ru nanoparticles supported on alumina. H/D isotopic exchange technique, complemented by DRIFTS analysis, was applied to study the kinetics of hydrogen adsorption/desorption on metallic Ru and hydrogen diffusivity on alumina, for catalysts presenting different size distributions of Ru nanoparticles. H atoms adsorbed on large Ru nanoparticles present higher mobility and they migrate on alumina via exchange with OH groups. A broad size distribution of Ru nanoparticles leads to synergy in the rate of ammonia synthesis, and also in hydrogen mobility. The mechanism of catalytic cooperation involves transfer of H atoms from large to small nanoparticles, where the reaction rate is promoted. Considering dynamic catalytic processes in the formulation of kinetic models is crucial for a more accurate description of processes and the development of large-scale processes.
中文翻译:

氧化铝负载的Ru纳米颗粒上氢吸附和迁移的动力学:对氨合成催化机理的影响
在这项工作中提供了有关氢吸附和迁移率的相关发现,以阐明由负载在氧化铝上的多分散Ru纳米颗粒催化的低温氨合成机理。采用氢/同位素交换技术,辅以DRIFTS分析,研究了Ru纳米粒子尺寸分布不同的催化剂对金属Ru的氢吸附/解吸动力学和在氧化铝上的氢扩散系数。吸附在大尺寸Ru纳米颗粒上的H原子具有较高的迁移率,并且它们通过与OH基团交换而在氧化铝上迁移。Ru纳米粒子的宽尺寸分布导致氨合成速率以及氢迁移率的协同作用。催化合作的机制涉及H原子从大颗粒到小颗粒的转移,反应速率提高的地方。在动力学模型的制定中考虑动态催化过程对于更准确地描述过程和开发大规模过程至关重要。
更新日期:2016-09-30
中文翻译:

氧化铝负载的Ru纳米颗粒上氢吸附和迁移的动力学:对氨合成催化机理的影响
在这项工作中提供了有关氢吸附和迁移率的相关发现,以阐明由负载在氧化铝上的多分散Ru纳米颗粒催化的低温氨合成机理。采用氢/同位素交换技术,辅以DRIFTS分析,研究了Ru纳米粒子尺寸分布不同的催化剂对金属Ru的氢吸附/解吸动力学和在氧化铝上的氢扩散系数。吸附在大尺寸Ru纳米颗粒上的H原子具有较高的迁移率,并且它们通过与OH基团交换而在氧化铝上迁移。Ru纳米粒子的宽尺寸分布导致氨合成速率以及氢迁移率的协同作用。催化合作的机制涉及H原子从大颗粒到小颗粒的转移,反应速率提高的地方。在动力学模型的制定中考虑动态催化过程对于更准确地描述过程和开发大规模过程至关重要。






























 京公网安备 11010802027423号
京公网安备 11010802027423号