当前位置:
X-MOL 学术
›
Adv. Synth. Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
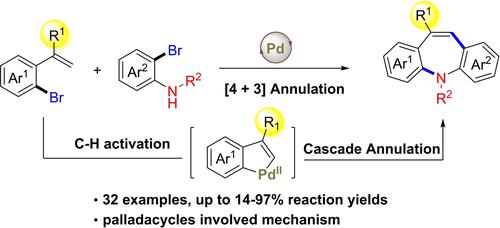
Synthesis of Dibenzo[b,f]azepines via Palladium-Catalyzed Cascade [4 + 3] Annulation of o-Alkenyl Bromoarenes and o-Bromoaniline Derivatives
Advanced Synthesis & Catalysis ( IF 4.4 ) Pub Date : 2023-06-12 , DOI: 10.1002/adsc.202300336 Xin-Chen Zhan 1 , Guo-Qiang Lin 2 , Jian-Guo Fu 3 , Xiaoming Ji 4 , Shu-Sheng Zhang 3 , Chen-Guo Feng 3
Advanced Synthesis & Catalysis ( IF 4.4 ) Pub Date : 2023-06-12 , DOI: 10.1002/adsc.202300336 Xin-Chen Zhan 1 , Guo-Qiang Lin 2 , Jian-Guo Fu 3 , Xiaoming Ji 4 , Shu-Sheng Zhang 3 , Chen-Guo Feng 3
Affiliation
|
A cascade [4+3] annulation of o-alkenyl bromoarenes and o-bromoaniline derivatives was described. Various dibenzo[b,f]azepines with substitutions on the 10/11 position were obtained in 14–97% yields. The synthetic versatility of this protocol is highlighted by the preparation of a precursor of the drug molecule oxcarbazepine, a gram-scale synthesis, and two product transformations. Unlike previous amination/Heck sequence, this cascade process is supposed to undergo a C(vinyl), C(aryl)-palladacycle involved pathway.
中文翻译:

通过钯催化级联 [4 + 3] 邻烯基溴芳烃和邻溴苯胺衍生物的环化合成二苯并[b,f]氮杂环庚烷
描述了邻-烯基溴芳烃和邻-溴苯胺衍生物的级联[ 4+3]环化。获得了在 10/11 位点上有取代的各种二苯并[ b , f ]氮杂卓化合物,产率为 14-97%。该方案的合成多功能性通过药物分子奥卡西平前体的制备、克级合成和两种产品转化来突出。与之前的胺化/Heck 序列不同,该级联过程应该经历 C(乙烯基)、C(芳基)-钯环参与途径。
更新日期:2023-06-12
中文翻译:

通过钯催化级联 [4 + 3] 邻烯基溴芳烃和邻溴苯胺衍生物的环化合成二苯并[b,f]氮杂环庚烷
描述了邻-烯基溴芳烃和邻-溴苯胺衍生物的级联[ 4+3]环化。获得了在 10/11 位点上有取代的各种二苯并[ b , f ]氮杂卓化合物,产率为 14-97%。该方案的合成多功能性通过药物分子奥卡西平前体的制备、克级合成和两种产品转化来突出。与之前的胺化/Heck 序列不同,该级联过程应该经历 C(乙烯基)、C(芳基)-钯环参与途径。































 京公网安备 11010802027423号
京公网安备 11010802027423号