Bioorganic Chemistry ( IF 4.5 ) Pub Date : 2023-06-13 , DOI: 10.1016/j.bioorg.2023.106663 Ning Yan 1 , Fei Xie 2 , Long-Qian Tang 1 , De-Feng Wang 1 , Xiang Li 1 , Chao Liu 1 , Zhao-Peng Liu 1
|
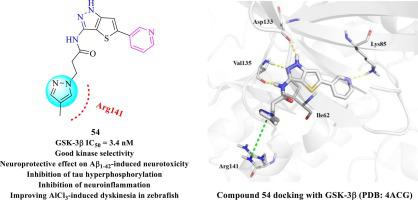
Glycogen synthase kinase 3β (GSK-3β) is a potential target for anti-Alzheimer’s disease (AD) drug development. In this study, a series of novel thieno[3,2-c]pyrazol-3-amine derivatives was synthesized and evaluated as potential GSK-3β inhibitors by structure-based drug design. The thieno[3,2-c]pyrazol-3-amine derivative 54 with a 4-methylpyrazole moiety which interacted with Arg141 by π-cation interaction was identified as a potent GSK-3β inhibitor with an IC50 of 3.4 nM and an acceptable kinase selectivity profile. In the rat primary cortical neurons, compound 54 showed neuroprotective effects on Aβ-induced neurotoxicity. Western blot analysis indicated that 54 inhibited GSK-3β by up-regulating the expression of phosphorylated GSK-3β at Ser9 and down-regulating the expression of phosphorylated GSK-3β at Tyr216. Meanwhile, 54 decreased tau phosphorylation at Ser396 in a dose-dependent way. In astrocytes and microglia cells, 54 inhibited the expression of inducible nitric oxide synthase (iNOS), indicating that 54 showed an anti-neuroinflammatory effect. In the AlCl3-induced zebrafish AD model, 54 significantly ameliorated the AlCl3-induced dyskinesia, demonstrating its anti-AD activity in vivo.
中文翻译:

噻吩并[3,2-c]吡唑-3-胺衍生物的合成和生物学评价作为有效的糖原合酶激酶3β抑制剂治疗阿尔茨海默病
糖原合成酶激酶 3β (GSK-3β) 是抗阿尔茨海默病 (AD) 药物开发的潜在靶标。在这项研究中,合成了一系列新型噻吩并[3,2- c ]吡唑-3-胺衍生物,并通过基于结构的药物设计评估其作为潜在的GSK-3β抑制剂的效果。具有 4-甲基吡唑部分的噻吩并[3,2- c ]吡唑-3-胺衍生物54通过 π-阳离子相互作用与 Arg141 相互作用,被鉴定为有效的 GSK-3β 抑制剂,IC 50为 3.4 nM,可接受的激酶选择性谱。在大鼠原代皮质神经元中,化合物54对 Aβ 诱导的神经毒性表现出神经保护作用。 Western blot分析表明54通过上调Ser9位点磷酸化GSK-3β的表达并下调Tyr216位点磷酸化GSK-3β的表达来抑制GSK-3β。同时, 54以剂量依赖性方式降低 Ser396 tau 磷酸化。在星形胶质细胞和小胶质细胞中, 54抑制诱导型一氧化氮合酶(iNOS)的表达,表明54表现出抗神经炎症作用。在AlCl 3诱导的斑马鱼AD模型中, 54显着改善了AlCl 3诱导的运动障碍,证明了其体内抗AD活性。






























 京公网安备 11010802027423号
京公网安备 11010802027423号