当前位置:
X-MOL 学术
›
Chem. Sci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Enantioselective synthesis of hydantoins by chiral acid-catalysed condensation of glyoxals and ureas
Chemical Science ( IF 7.6 ) Pub Date : 2023-06-13 , DOI: 10.1039/d3sc01656k Sushant Aryal 1 , Christopher A Hone 2 , Matthew I J Polson 1 , Daniel J Foley 1, 3
Chemical Science ( IF 7.6 ) Pub Date : 2023-06-13 , DOI: 10.1039/d3sc01656k Sushant Aryal 1 , Christopher A Hone 2 , Matthew I J Polson 1 , Daniel J Foley 1, 3
Affiliation
|
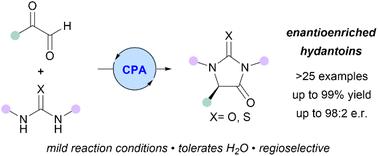
Hydantoins are important scaffolds in natural products and pharmaceuticals, with only a few synthetic strategies available for their asymmetric preparation. We herein describe a single-step enantioselective synthesis of 5-monosubstituted hydantoins via condensation of glyoxals and ureas in the presence of a chiral phosphoric acid at room temperature. Products were formed in up to 99% yield and 98 : 2 e.r. Using mechanistic and kinetic studies, including time course 1H NMR monitoring, we revealed that the reaction likely proceeds via face-selective protonation of an enol-type intermediate.
中文翻译:

手性酸催化乙二醛和脲缩合对映选择性合成乙内酰脲
乙内酰脲是天然产物和药物中的重要支架,但只有少数合成策略可用于其不对称制备。我们在此描述了在手性磷酸存在下在室温下通过乙二醛和脲的缩合来一步对映选择性合成5-单取代乙内酰脲。产物的产率高达 99%,比率为 98:2。通过机械和动力学研究,包括时程1 H NMR 监测,我们发现该反应可能是通过烯醇型中间体的面选择性质子化进行的。
更新日期:2023-06-13
中文翻译:

手性酸催化乙二醛和脲缩合对映选择性合成乙内酰脲
乙内酰脲是天然产物和药物中的重要支架,但只有少数合成策略可用于其不对称制备。我们在此描述了在手性磷酸存在下在室温下通过乙二醛和脲的缩合来一步对映选择性合成5-单取代乙内酰脲。产物的产率高达 99%,比率为 98:2。通过机械和动力学研究,包括时程1 H NMR 监测,我们发现该反应可能是通过烯醇型中间体的面选择性质子化进行的。































 京公网安备 11010802027423号
京公网安备 11010802027423号