当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Divergent Access to Chiral C2- and C3-Alkylated Pyrrolidines by Catalyst-Tuned Regio- and Enantioselective C(sp3)–C(sp3) Coupling
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2023-06-12 , DOI: 10.1021/jacs.3c03900 Xuchao Wang 1 , Jing Xue 1 , Zi-Qiang Rong 1
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2023-06-12 , DOI: 10.1021/jacs.3c03900 Xuchao Wang 1 , Jing Xue 1 , Zi-Qiang Rong 1
Affiliation
|
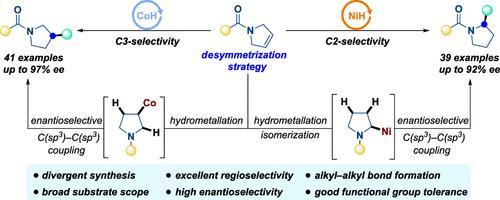
Novel-substituted pyrrolidine derivatives are widely used in drugs and bioactive molecules. The efficient synthesis of these valuable skeletons, especially enantiopure derivatives, is still recognized as a key bottleneck to overcome in chemical synthesis. Herein, we report a highly efficient catalyst-tuned regio- and enantioselective hydroalkylation reaction for the divergent synthesis of chiral C2- and C3-alkylated pyrrolidines through desymmetrization of the readily available 3-pyrrolines. The catalytic system consists of CoBr2 with a modified bisoxazoline (BOX) ligand, which can achieve the asymmetric C(sp3)–C(sp3) coupling via the distal stereocontrol, providing a series of C3-alkylated pyrrolidines in high efficiency. Moreover, the nickel catalytic system allows the enantioselective hydroalkylation to synthesize the C2-alkylated pyrrolidines through the tandem alkene isomerization/hydroalkylation reaction. This divergent method uses readily available catalysts, chiral BOX ligands, and reagents, delivering enantioenriched 2-/3-alkyl substituted pyrrolidines with excellent regio- and enantioselectivity (up to 97% ee). We also demonstrate the compatibility of this transformation with complex substrates derived from a series of drugs and bioactive molecules in good efficiency, which offers a distinct entry to more functionalized chiral N-heterocycles.
中文翻译:

通过催化剂调节的区域选择性和对映选择性 C(sp3)–C(sp3) 偶联不同地获得手性 C2- 和 C3- 烷基化吡咯烷
新型取代吡咯烷衍生物广泛应用于药物和生物活性分子。这些有价值的骨架,特别是对映体纯衍生物的有效合成,仍然被认为是化学合成中需要克服的关键瓶颈。在此,我们报道了一种高效催化剂调节的区域选择性和对映选择性加氢烷基化反应,用于通过容易获得的 3-吡咯啉的去对称化来不同合成手性 C2-和 C3-烷基化吡咯烷。该催化体系由CoBr 2和修饰的双恶唑啉(BOX)配体组成,可以通过远端立体控制实现不对称C(sp 3)–C(sp 3)偶联,高效地提供一系列C3烷基化吡咯烷。此外,镍催化体系允许对映选择性加氢烷基化通过串联烯烃异构化/加氢烷基化反应合成C2-烷基化吡咯烷。这种不同的方法使用现成的催化剂、手性 BOX 配体和试剂,提供对映体富集的 2-/3-烷基取代的吡咯烷,具有优异的区域和对映选择性(高达 97% ee)。我们还证明了这种转化与衍生自一系列药物和生物活性分子的复杂底物的相容性良好,这为更多功能化的手性N-杂环提供了独特的途径。
更新日期:2023-06-12
中文翻译:

通过催化剂调节的区域选择性和对映选择性 C(sp3)–C(sp3) 偶联不同地获得手性 C2- 和 C3- 烷基化吡咯烷
新型取代吡咯烷衍生物广泛应用于药物和生物活性分子。这些有价值的骨架,特别是对映体纯衍生物的有效合成,仍然被认为是化学合成中需要克服的关键瓶颈。在此,我们报道了一种高效催化剂调节的区域选择性和对映选择性加氢烷基化反应,用于通过容易获得的 3-吡咯啉的去对称化来不同合成手性 C2-和 C3-烷基化吡咯烷。该催化体系由CoBr 2和修饰的双恶唑啉(BOX)配体组成,可以通过远端立体控制实现不对称C(sp 3)–C(sp 3)偶联,高效地提供一系列C3烷基化吡咯烷。此外,镍催化体系允许对映选择性加氢烷基化通过串联烯烃异构化/加氢烷基化反应合成C2-烷基化吡咯烷。这种不同的方法使用现成的催化剂、手性 BOX 配体和试剂,提供对映体富集的 2-/3-烷基取代的吡咯烷,具有优异的区域和对映选择性(高达 97% ee)。我们还证明了这种转化与衍生自一系列药物和生物活性分子的复杂底物的相容性良好,这为更多功能化的手性N-杂环提供了独特的途径。




















































 京公网安备 11010802027423号
京公网安备 11010802027423号