当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Isolable Analogues of the Breslow Intermediate Derived from Chiral Triazolylidene Carbenes
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2012-03-28 , DOI: 10.1021/ja302031v Daniel A DiRocco 1 , Kevin M Oberg , Tomislav Rovis
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2012-03-28 , DOI: 10.1021/ja302031v Daniel A DiRocco 1 , Kevin M Oberg , Tomislav Rovis
Affiliation
|
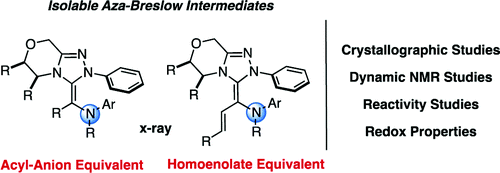
Since Breslow's initial report on the thiamine mode of action, the study of catalytic acyl carbanion processes has been an area of immense interest. With the advent of azolylidene catalysis, a plethora of reactivtiy has been harnessed, but the crucial nucleophilic intermediate proposed by Breslow had never been isolated or fully characterized. Herein, we report the isolation and full characterization of nitrogen analogues of the Breslow intermediate. Both stable and catalytically relevant, these species provide a model system for the study of acyl carbanion and homoenolate processes catalyzed by triazolylidene carbenes.
中文翻译:

手性三唑基卡宾衍生的 Breslow 中间体的可分离类似物
自从 Breslow 首次报告硫胺素作用模式以来,催化酰基碳负离子过程的研究一直是人们非常感兴趣的领域。随着亚唑基催化的出现,大量的反应活性被利用,但布雷斯洛提出的关键亲核中间体从未被分离或完全表征。在此,我们报告了 Breslow 中间体的氮类似物的分离和完整表征。这些物种既稳定又具有催化相关性,为研究三唑基卡宾催化的酰基碳负离子和高烯醇化物过程提供了模型系统。
更新日期:2012-03-28
中文翻译:

手性三唑基卡宾衍生的 Breslow 中间体的可分离类似物
自从 Breslow 首次报告硫胺素作用模式以来,催化酰基碳负离子过程的研究一直是人们非常感兴趣的领域。随着亚唑基催化的出现,大量的反应活性被利用,但布雷斯洛提出的关键亲核中间体从未被分离或完全表征。在此,我们报告了 Breslow 中间体的氮类似物的分离和完整表征。这些物种既稳定又具有催化相关性,为研究三唑基卡宾催化的酰基碳负离子和高烯醇化物过程提供了模型系统。































 京公网安备 11010802027423号
京公网安备 11010802027423号