Tetrahedron ( IF 2.1 ) Pub Date : 2023-06-10 , DOI: 10.1016/j.tet.2023.133515 Chun-Yu Liu , Yu Chen , Xian-Hua Pan , Fa-Zhan Liang , Zhao-Qi Liu
|
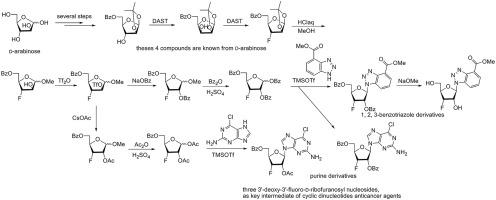
An alternative synthesis of 3′-deoxy-3′-fluoro-d-ribofuranosyl nucleoside from d-arabinose is disclosed. Inversion of configuration at C3 on d-arabinoside promoted by DAST reagent is utilized to form lyxo-configured furanoside. After a sequence of fluorination, acidic methanolysis, triflation and displacement by OBz. 3′-deoxy-3′-fluoro-d-ribofuranoside is obtained, which is then transformed to 1-O-benzoyl derivative. The synthesis of three 3′-deoxy-3′-fluoro-d-ribofuranosyl nucleosides, which can be served as fragment of antitumor active cyclic dinucleotide is finally completed through Vorbrüggen glycosylation .
中文翻译:

抗肿瘤活性环状二核苷酸的重要片段3'-脱氧-3'-氟-d-呋喃核苷的替代合成
公开了从d-阿拉伯糖合成 3'-脱氧-3'-氟-d-呋喃核苷的替代方法。由 DAST 试剂促进的d-阿拉伯糖苷的 C3 构型反转用于形成 lyxo-构型的呋喃糖苷。经过一系列氟化、酸性甲醇分解、三氟甲磺酸化和 OBz 置换后。得到3'-脱氧-3'-氟-d-呋喃核糖苷,然后转化为1- O-苯甲酰基衍生物。最终通过Vorbrüggen糖基化完成三个3'-脱氧-3'-氟-d-呋喃核糖核苷的合成,可作为抗肿瘤活性环状二核苷酸的片段。






























 京公网安备 11010802027423号
京公网安备 11010802027423号