当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Formal Synthesis of (±)-Methyl Rocaglate Using an Unprecedented Acetyl Bromide Mediated Nazarov Reaction
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2012-03-28 , DOI: 10.1021/ja300900p Philip Magnus 1 , Wesley A. Freund 1 , Eric J. Moorhead 1 , Trevor Rainey 1
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2012-03-28 , DOI: 10.1021/ja300900p Philip Magnus 1 , Wesley A. Freund 1 , Eric J. Moorhead 1 , Trevor Rainey 1
Affiliation
|
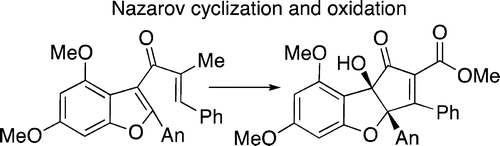
To date the prototype Nazarov cyclization of a cross-conjugated pentadienone to the core structure of the rocaglate natural products has not been successful (9 into 12). It has been found that this conversion can be achieved by the use of acetylbromide in excellent yield and results in a strategically very direct route to these antitumor agents.
中文翻译:

使用前所未有的乙酰溴介导的 Nazarov 反应正式合成 (±)-Methyl Rocaglate
迄今为止,交叉共轭戊二烯酮与 rocaglate 天然产物核心结构的原型 Nazarov 环化尚未成功(9 到 12)。已经发现这种转化可以通过以极好的收率使用乙酰溴来实现,并导致获得这些抗肿瘤剂的战略性非常直接的途径。
更新日期:2012-03-28
中文翻译:

使用前所未有的乙酰溴介导的 Nazarov 反应正式合成 (±)-Methyl Rocaglate
迄今为止,交叉共轭戊二烯酮与 rocaglate 天然产物核心结构的原型 Nazarov 环化尚未成功(9 到 12)。已经发现这种转化可以通过以极好的收率使用乙酰溴来实现,并导致获得这些抗肿瘤剂的战略性非常直接的途径。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号