当前位置:
X-MOL 学术
›
ACS Biomater. Sci. Eng.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
ALA/Ag2S/MnO2 Hybrid Nanoparticles for Near-Infrared Image-Guided Long-Wavelength Phototherapy of Breast Cancer
ACS Biomaterials Science & Engineering ( IF 5.4 ) Pub Date : 2023-06-09 , DOI: 10.1021/acsbiomaterials.3c00105 Mahshid Hashemkhani 1 , Eda Celikbas 2 , Minahil Khan 3 , Alphan Sennaroglu 1, 3, 4 , Havva Yagci Acar 1, 2
ACS Biomaterials Science & Engineering ( IF 5.4 ) Pub Date : 2023-06-09 , DOI: 10.1021/acsbiomaterials.3c00105 Mahshid Hashemkhani 1 , Eda Celikbas 2 , Minahil Khan 3 , Alphan Sennaroglu 1, 3, 4 , Havva Yagci Acar 1, 2
Affiliation
|
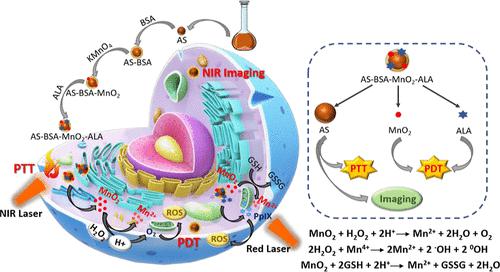
The combination of photothermal therapy (PTT) and photodynamic therapy (PDT) based on temperature increase and the formation of reactive oxygen species (ROS), respectively, is an exciting avenue to provide local and improved therapy of tumors with minimal off-site toxicity. 5-Aminolevulinic acid (ALA) is one of the most popular PDT pro-drugs, and its efficiency improves significantly when delivered to tumors with nanoparticles (NPs). But the tumor site’s hypoxic environment is a handicap for the oxygen-consuming PDT process. In this work, highly stable, small, theranostic NPs composed of Ag2S quantum dots and MnO2, electrostatically loaded with ALA, were developed for enhanced PDT/PTT combination of tumors. MnO2 catalyzes endogenous H2O2 to O2 conversion and glutathione depletion, enhancing ROS generation and ALA-PDT efficiency. Ag2S quantum dots (AS QDs) conjugated with bovine serum albumin (BSA) support MnO2 formation and stabilization around Ag2S. AS-BSA-MnO2 provided a strong intracellular near-infrared (NIR) signal and increased the solution temperature by 15 °C upon laser irradiation at 808 nm (215 mW, 10 mg/mL), proving the hybrid NP as an optically trackable, long-wavelength PTT agent. In the in vitro studies, no significant cytotoxicity was observed in the absence of laser irradiation in healthy (C2C12) or breast cancer cell lines (SKBR3 and MDA-MB-231). The most effective phototoxicity was observed when AS-BSA-MnO2-ALA-treated cells were co-irradiated for 5 min with 640 nm (300 mW) and 808 nm (700 mW) due to enhanced ALA-PDT combined with PTT. The viability of cancer cells decreased to approximately 5–10% at 50 μg/mL [Ag], corresponding to 1.6 mM [ALA], whereas at the same concentration, individual PTT and PDT treatments decreased the viability to 55–35%, respectively. The late apoptotic death of the treated cells was mostly correlated with high ROS levels and lactate dehydrogenase. Overall, these hybrid NPs overcome tumor hypoxia, deliver ALA to tumor cells, and provide both NIR tracking and enhanced PDT + PTT combination therapy upon short, low-dose co-irradiation at long wavelengths. These agents that may be utilized for treating other cancer types are also highly suitable for in vivo investigations.
中文翻译:

ALA/Ag2S/MnO2 混合纳米粒子用于乳腺癌近红外图像引导长波长光疗
分别基于温度升高和活性氧(ROS)形成的光热疗法(PTT)和光动力疗法(PDT)的组合是一种令人兴奋的途径,可以以最小的异位毒性提供局部和改进的肿瘤治疗。5-氨基乙酰丙酸 (ALA) 是最流行的 PDT 前药之一,当通过纳米粒子 (NP) 递送至肿瘤时,其效率显着提高。但肿瘤部位的缺氧环境是耗氧PDT过程的一个障碍。在这项工作中,开发了由Ag 2 S量子点和MnO 2组成的高度稳定的小型治疗诊断纳米颗粒,静电负载有ALA,用于增强肿瘤的PDT/PTT组合。MnO 2催化内源H 2O 2到 O 2 的转化和谷胱甘肽的消耗,增强 ROS 的生成和 ALA-PDT 效率。与牛血清白蛋白 (BSA) 结合的Ag 2 S 量子点 (AS QD) 支持Ag 2 S周围 MnO 2 的形成和稳定。AS-BSA-MnO 2提供了强烈的细胞内近红外 (NIR) 信号,并在 808 nm(215 mW,10 mg/mL)激光照射下将溶液温度提高了 15 °C,证明了混合 NP 作为光学可追踪的长波长 PTT 剂。在体外研究中,在健康细胞系(C2C12)或乳腺癌细胞系(SKBR3 和 MDA-MB-231)中,在没有激光照射的情况下,没有观察到明显的细胞毒性。当 AS-BSA-MnO 2时观察到最有效的光毒性由于增强的 ALA-PDT 与 PTT 相结合,用 640 nm (300 mW) 和 808 nm (700 mW) 联合照射 -ALA 处理的细胞 5 分钟。50 μg/mL [Ag](相当于 1.6 mM [ALA])时,癌细胞的活力下降至约 5-10%,而在相同浓度下,单独的 PTT 和 PDT 治疗将活力分别降低至 55-35% 。处理细胞的晚期凋亡主要与高ROS水平和乳酸脱氢酶相关。总体而言,这些混合纳米颗粒克服了肿瘤缺氧,将 ALA 输送到肿瘤细胞,并在长波长短、低剂量联合照射下提供近红外追踪和增强的 PDT + PTT 联合治疗。这些可用于治疗其他癌症类型的药物也非常适合体内研究。
更新日期:2023-06-09
中文翻译:

ALA/Ag2S/MnO2 混合纳米粒子用于乳腺癌近红外图像引导长波长光疗
分别基于温度升高和活性氧(ROS)形成的光热疗法(PTT)和光动力疗法(PDT)的组合是一种令人兴奋的途径,可以以最小的异位毒性提供局部和改进的肿瘤治疗。5-氨基乙酰丙酸 (ALA) 是最流行的 PDT 前药之一,当通过纳米粒子 (NP) 递送至肿瘤时,其效率显着提高。但肿瘤部位的缺氧环境是耗氧PDT过程的一个障碍。在这项工作中,开发了由Ag 2 S量子点和MnO 2组成的高度稳定的小型治疗诊断纳米颗粒,静电负载有ALA,用于增强肿瘤的PDT/PTT组合。MnO 2催化内源H 2O 2到 O 2 的转化和谷胱甘肽的消耗,增强 ROS 的生成和 ALA-PDT 效率。与牛血清白蛋白 (BSA) 结合的Ag 2 S 量子点 (AS QD) 支持Ag 2 S周围 MnO 2 的形成和稳定。AS-BSA-MnO 2提供了强烈的细胞内近红外 (NIR) 信号,并在 808 nm(215 mW,10 mg/mL)激光照射下将溶液温度提高了 15 °C,证明了混合 NP 作为光学可追踪的长波长 PTT 剂。在体外研究中,在健康细胞系(C2C12)或乳腺癌细胞系(SKBR3 和 MDA-MB-231)中,在没有激光照射的情况下,没有观察到明显的细胞毒性。当 AS-BSA-MnO 2时观察到最有效的光毒性由于增强的 ALA-PDT 与 PTT 相结合,用 640 nm (300 mW) 和 808 nm (700 mW) 联合照射 -ALA 处理的细胞 5 分钟。50 μg/mL [Ag](相当于 1.6 mM [ALA])时,癌细胞的活力下降至约 5-10%,而在相同浓度下,单独的 PTT 和 PDT 治疗将活力分别降低至 55-35% 。处理细胞的晚期凋亡主要与高ROS水平和乳酸脱氢酶相关。总体而言,这些混合纳米颗粒克服了肿瘤缺氧,将 ALA 输送到肿瘤细胞,并在长波长短、低剂量联合照射下提供近红外追踪和增强的 PDT + PTT 联合治疗。这些可用于治疗其他癌症类型的药物也非常适合体内研究。





















































 京公网安备 11010802027423号
京公网安备 11010802027423号