Journal of Energy Chemistry ( IF 14.0 ) Pub Date : 2023-06-08 , DOI: 10.1016/j.jechem.2023.05.033 Zhengrong Liu , Jun Zhou , Yueyue Sun , Xiangling Yue , Jiaming Yang , Lei Fu , Qinyuan Deng , Hongfei Zhao , Chaofan Yin , Kai Wu
|
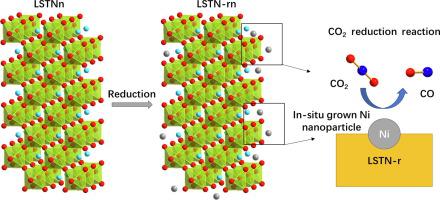
Solid oxide electrolysis cell (SOEC) could be a potential technology to afford chemical storage of renewable electricity by converting water and carbon dioxide. In this work, we present the Ni-doped layered perovskite oxides, (La4Srn−4)0.9Ti0.9nNi0.1nO3n+2 with n = 5, 8, and 12 (LSTNn) for application as catalysts of CO2 electrolysis with the exsolution of Ni nanoparticles through a simple in-situ growth method. It is found that the density, size, and distribution of exsolved Ni nanoparticles are determined by the number of n in LSTNn due to the different stack structures of TiO6 octahedra along the c axis. The Ni doping in LSTNn significantly improved the electrochemical activity by increasing oxygen vacancies, and the Ni metallic nanoparticles afford much more active sites. The results show that LSTNn cathodes can successfully be manipulated the activity by controlling both the n number and Ni exsolution. Among these LSTNn (n = 5, 8, and 12), LSTN8 renders a higher activity for electrolysis of CO2 with a current density of 1.50A cm−2@2.0 V at 800 °C It is clear from these results that the number of n in (La4Srn−4)0.9Ti0.9nNi0.1nO3n+2 with Ni-doping is a key factor in controlling the electrochemical performance and catalytic activity in SOEC.
中文翻译:

调整缺陷工程层状钙钛矿氧化物中纳米颗粒的溶出,以实现高效的二氧化碳电解
固体氧化物电解池(SOEC)可能是一种通过转化水和二氧化碳来化学存储可再生电力的潜在技术。在这项工作中,我们提出了 Ni 掺杂的层状钙钛矿氧化物 (La 4 Sr n −4 ) 0.9 Ti 0.9 n Ni 0.1 n O 3 n +2其中n = 5、8 和 12 (LSTNn) 作为催化剂的应用CO 2的通过简单的原位生长方法进行电解,使镍纳米颗粒溶出。研究发现,由于TiO 6 八面体沿c轴的堆叠结构不同,溶出的Ni纳米粒子的密度、尺寸和分布由LSTNn中的n数决定。LSTNn 中的 Ni 掺杂通过增加氧空位显着提高了电化学活性,并且 Ni 金属纳米颗粒提供了更多的活性位点。结果表明,通过控制 n 数和 Ni 溶出量,可以成功地控制 LSTNn 阴极的活性。在这些LSTNn(n = 5、8和12)中,LSTN8在电流密度为1.50A cm -2时对CO 2电解具有更高的活性@2.0 V at 800 °C 从这些结果可以清楚地看出,掺杂 Ni 的 (La 4 Sr n −4 ) 0.9 Ti 0.9 n Ni 0.1 n O 3 n +2中的 n 数是控制SOEC 的电化学性能和催化活性。

































 京公网安备 11010802027423号
京公网安备 11010802027423号