当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Engineering Single-Atom Electrocatalysts for Enhancing Kinetics of Acidic Volmer Reaction
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2023-06-07 , DOI: 10.1021/jacs.2c13418 Hao Cao , Qilun Wang 1 , Zisheng Zhang 2 , Hui-Min Yan , Hongyan Zhao , Hong Bin Yang 1 , Bin Liu 1, 3 , Jun Li 4 , Yang-Gang Wang
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2023-06-07 , DOI: 10.1021/jacs.2c13418 Hao Cao , Qilun Wang 1 , Zisheng Zhang 2 , Hui-Min Yan , Hongyan Zhao , Hong Bin Yang 1 , Bin Liu 1, 3 , Jun Li 4 , Yang-Gang Wang
Affiliation
|
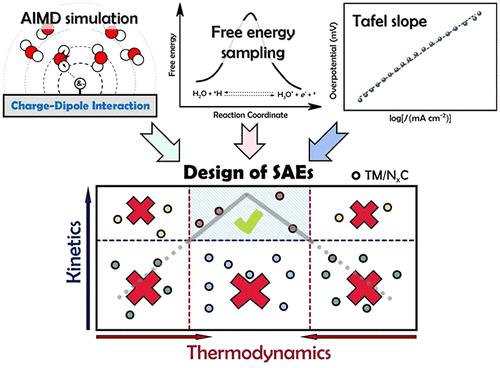
The design of active and low-cost electrocatalyst for hydrogen evolution reaction (HER) is the key to achieving a clean hydrogen energy infrastructure. The most successful design principle of hydrogen electrocatalyst is the activity volcano plot, which is based on Sabatier principle and has been used to understand the exceptional activity of noble metal and design of metal alloy catalysts. However, this application of volcano plot in designing single-atom electrocatalysts (SAEs) on nitrogen doped graphene (TM/N4C catalysts) for HER has been less successful due to the nonmetallic nature of the single metal atom site. Herein, by performing ab initio molecular dynamics simulations and free energy calculations on a series of SAEs systems (TM/N4C with TM = 3d, 4d, or 5d metals), we find that the strong charge–dipole interaction between the negatively charged *H intermediate and the interfacial H2O molecules could alter the transition path of the acidic Volmer reaction and dramatically raise its kinetic barrier, despite its favorable adsorption free energy. Such kinetic hindrance is also experimentally confirmed by electrochemical measurements. By combining the hydrogen adsorption free energy and the physics of competing interfacial interactions, we propose a unifying design principle for engineering the SAEs used for hydrogen energy conversion, which incorporates both thermodynamic and kinetic considerations and allows going beyond the activity volcano model.
中文翻译:

用于增强酸性 Volmer 反应动力学的工程单原子电催化剂
用于析氢反应(HER)的活性且低成本的电催化剂的设计是实现清洁氢能源基础设施的关键。氢电催化剂最成功的设计原理是活性火山图,它基于萨巴蒂尔原理,已用于了解贵金属的特殊活性和金属合金催化剂的设计。然而,由于单金属原子位点的非金属性质,火山图在氮掺杂石墨烯(TM/N 4 C 催化剂)上设计用于 HER 的单原子电催化剂(SAE)的应用不太成功。在此,通过对一系列 SAE 系统(TM/N 4C 与 TM = 3d、4d 或 5d 金属),我们发现带负电的 *H 中间体和界面 H 2 O分子之间的强电荷-偶极相互作用可以改变酸性 Volmer 反应的过渡路径并显着提高其反应速度。动势垒,尽管其具有有利的吸附自由能。这种动力学阻碍也通过电化学测量得到了实验证实。通过结合氢吸附自由能和竞争界面相互作用的物理原理,我们提出了用于氢能转换的 SAE 工程的统一设计原则,该原则结合了热力学和动力学考虑,并允许超越活动火山模型。
更新日期:2023-06-07
中文翻译:

用于增强酸性 Volmer 反应动力学的工程单原子电催化剂
用于析氢反应(HER)的活性且低成本的电催化剂的设计是实现清洁氢能源基础设施的关键。氢电催化剂最成功的设计原理是活性火山图,它基于萨巴蒂尔原理,已用于了解贵金属的特殊活性和金属合金催化剂的设计。然而,由于单金属原子位点的非金属性质,火山图在氮掺杂石墨烯(TM/N 4 C 催化剂)上设计用于 HER 的单原子电催化剂(SAE)的应用不太成功。在此,通过对一系列 SAE 系统(TM/N 4C 与 TM = 3d、4d 或 5d 金属),我们发现带负电的 *H 中间体和界面 H 2 O分子之间的强电荷-偶极相互作用可以改变酸性 Volmer 反应的过渡路径并显着提高其反应速度。动势垒,尽管其具有有利的吸附自由能。这种动力学阻碍也通过电化学测量得到了实验证实。通过结合氢吸附自由能和竞争界面相互作用的物理原理,我们提出了用于氢能转换的 SAE 工程的统一设计原则,该原则结合了热力学和动力学考虑,并允许超越活动火山模型。































 京公网安备 11010802027423号
京公网安备 11010802027423号