Talanta ( IF 5.6 ) Pub Date : 2023-06-07 , DOI: 10.1016/j.talanta.2023.124774 Ming Cai 1 , Qian Zhang 1 , Lili Lan 1 , Wanyang Sun 2 , Hong Zhang 3 , Guoxiang Sun 1
|
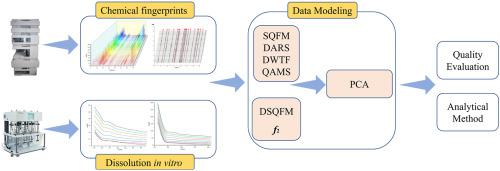
In recent years, traditional analytical methods have failed to meet the widespread use of multi-component Chinese pharmaceutical formulations. To solve this problem, this study proposed a comprehensive analytical strategy using compound liquorice tablets (CLTs) as an example, both in terms of chemical quality and dissolution curve consistency. Firstly, the peak purity of the two wavelengths was checked using dual-wavelength absorbance coefficient ratio spectra (DARS) to avoid the fingerprint bias caused by peak purity. Secondly, liquid-phase dual-wavelength tandem fingerprint (DWTF) of 38 batches of CLTs was established for the first time. The two analytical methods were also evaluated using the systematically quantified fingerprint method (SQFM), and the 38 batches of samples were classified into two grades with good quality consistency. Quantitative analysis of the five markers of CLTs was performed simultaneously using the standard curve method (SCM) and the quantitative analysis of multiple components by single marker (QAMS). The results showed no significant differences between the two analytical methods (p > 0.5). In addition, the in vitro dissolution of CLTs in two media (pure water and pH = 4.5 medium) was determined by the total UV fingerprint dissolution assay. The similarity of the dissolution curves was also analyzed by combining the f2 factor and the dissolution-systematically quantified fingerprint method (DSQFM). The result showed that most of the samples had f2 > 50 and Pm satisfied the range of 70–130%. Finally, a principal component analysis (PCA) model was developed to combine the evaluation parameters of chemical fingerprint and dissolution curves for comprehensive analysis of the samples. In this study, a chromatographic and dissolution-based quality analysis method was proposed, which effectively overcomes the shortcomings of previous analytical methods and provides a scientific analytical method for the quality control of natural drugs.
中文翻译:

通过系统量化指纹法从所有化学指纹图谱和综合溶出曲线的相似性全面评估复方甘草片的质量一致性
近年来,传统的分析方法已不能满足多组分中药制剂的广泛使用。为解决这一问题,本研究以复方甘草片 (CLT) 为例,提出了一种在化学质量和溶出曲线一致性方面的综合分析策略。首先,使用双波长吸收系数比光谱(DARS)检查两个波长的峰纯度,以避免峰纯度引起的指纹偏差。其次,首次建立了38批次CLTs的液相双波长串联指纹图谱(DWTF)。还使用系统定量指纹法(SQFM)对两种分析方法进行了评价,将38批样品分为两个等级,质量一致性良好。使用标准曲线法(SCM)和单标记多组分定量分析(QAMS)同时对CLTs的五种标记物进行定量分析。结果表明两种分析方法之间没有显着差异 (p > 0.5)。除此之外CLT 在两种介质(纯水和 pH = 4.5 介质)中的体外溶解通过总 UV 指纹溶解测定法确定。还通过结合f 2因子和溶出系统量化指纹法 (DSQFM)分析了溶出曲线的相似性。结果表明,大部分样品具有f 2 > 50和P m满足 70-130% 的范围。最后,开发了主成分分析(PCA)模型,结合化学指纹图谱和溶出曲线的评价参数对样品进行综合分析。本研究提出了一种基于色谱和溶出度的质量分析方法,有效克服了以往分析方法的不足,为天然药物的质量控制提供了一种科学的分析方法。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号