European Journal of Medicinal Chemistry ( IF 6.0 ) Pub Date : 2023-06-07 , DOI: 10.1016/j.ejmech.2023.115548 Yogesh Nandurkar 1 , Manish R Bhoye 2 , Deepika Maliwal 3 , Raghuvir R S Pissurlenkar 4 , Abhijit Chavan 5 , Sushma Katade 6 , Pravin C Mhaske 5
|
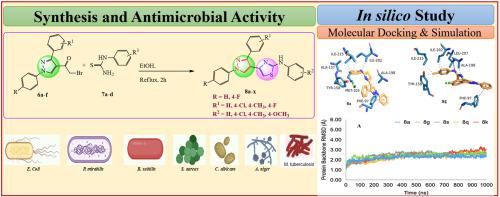
A new series of N-aryl-4-(1,3-diaryl-1H-pyrazol-4-yl)thiazol-2-amine, (8a-x) have been synthesized by a cyclo-condensation reaction of 2-bromo-1-(1,3-diphenyl-1H-pyrazol-4-yl)ethanone (6a-f) with N-aryl thiourea, (7a-d). The structure of newly synthesized N-aryl-4-(1,3-diaryl-1H-pyrazol-4-yl)thiazol-2-amine, (8a-x) derivatives was analyzed by 1H NMR, 13C NMR and Mass spectral analysis. The compounds 8a-x were screened for in vitro antimicrobial activity against Escherichia coli, Proteus mirabilis, Bacillus subtilis, Staphylococcus aureus, Candida albicans and Aspergillus niger. and antitubercular activity against M. tuberculosis H37Rv strain. Among the twenty-four pyrazolyl-thiazole derivatives, six compounds 8a, 8b, 8j, 8n, 8o and 8s showed good activity against S. aureus. Against A. niger, all synthesized derivatives showed good antifungal activity. Fifteen pyrazolyl-thiazole derivatives 8a, 8f, 8g, 8h, 8j, 8k, 8n, 8o, 8p, 8q, 8r, 8s, 8t, 8w and 8x showed good antitubercular activity with MIC 1.80–7.34 μM (0.8–3.12 μg/mL), these derivatives have showed more activity than the drugs isoniazid and ethambutol. The active compounds were further screened for cytotoxicity activity against the mouse embryonic fibroblast cells (3t3l1) cell lines at 12.5 and 25 μg/mL concentrations and found less or non-cytotoxicity. To know the plausible mode of action, the synthesized pyrazolyl-thiazole derivatives were studied for pharmacokinetics, toxicity profiles and binding interactions along with an in-depth analysis of structural dynamics and integrity using prolonged molecular dynamics (MD) simulation. The compounds have shown significant docking scores in the range of −7.98 to −5.52 and −9.44 to −7.2 kcal/mol with the M. tuberculosis enoyl reductase (M. tb. InhA) and C. albicans sterol 14-α demethylase (C. ab. CYP51), respectively. Thus, the significant antifungal and antitubercular activity of N-aryl-4-(1,3-diaryl-1H-pyrazol-4-yl)thiazol-2-amine, (8a-x) derivatives incited that, these scaffolds could assist in the development of lead compounds to treat fungal and antitubercular infections.
中文翻译:

作为潜在抗真菌和抗结核药物的新型 N-苯基-4-(1,3-二芳基-1H-吡唑-4-基)噻唑-2-胺衍生物的合成、生物筛选和计算机研究
通过2-溴的环缩合反应合成了一系列新的N-芳基-4-(1,3-二芳基-1 H-吡唑-4-基)噻唑-2-胺, ( 8a-x ) -1-(1,3-二苯基-1H-吡唑-4-基)乙酮( 6a-f )与N-芳基硫脲,( 7a-d )。新合成的N-芳基-4-(1,3-二芳基-1H-吡唑-4-基)噻唑-2-胺,( 8a-x )衍生物的结构通过1 H NMR、13 C NMR 和质谱分析。筛选了化合物8a-x对大肠杆菌、奇异变形杆菌、枯草芽孢杆菌、金黄色葡萄球菌、白色念珠菌和黑曲霉的体外抗菌活性。以及针对结核分枝杆菌H37Rv 菌株的抗结核活性。在24种吡唑基噻唑衍生物中,6种化合物8a、8b、8j、8n、8o和8s显示出良好的抗金黄色葡萄球菌活性。针对黑曲霉,所有合成的衍生物均表现出良好的抗真菌活性。15 个吡唑基噻唑衍生物8a、8f、8g、8h、8j、8k、8n、8o、8p、8q、8r、8s、8t、8w和8x显示出良好的抗结核活性, MIC 为 1.80–7.34 μM (0.8–3.12 μg/ mL),这些衍生物比药物异烟肼和乙胺丁醇表现出更高的活性。进一步筛选活性化合物在 12.5 和 25 μg/mL 浓度下对小鼠胚胎成纤维细胞 ( 3t3l1 ) 细胞系的细胞毒性活性,发现细胞毒性较小或无细胞毒性。为了了解合理的作用模式,研究了合成的吡唑基噻唑衍生物的药代动力学、毒性特征和结合相互作用,并使用长时间的分子动力学 (MD) 模拟对结构动力学和完整性进行了深入分析。这些化合物与结核分枝杆菌烯酰还原酶 ( M. tb. InhA) 的对接得分在 -7.98 至 -5.52 和 -9.44 至 -7.2 kcal/mol 范围内,分别为白色念珠菌甾醇14-α脱甲基酶(C. ab. CYP51)。因此,N-芳基-4-(1,3-二芳基-1H-吡唑-4-基)噻唑-2-胺( 8a-x )衍生物的显着抗真菌和抗结核活性表明,这些支架可以帮助开发治疗真菌和抗结核感染的先导化合物。






























 京公网安备 11010802027423号
京公网安备 11010802027423号