当前位置:
X-MOL 学术
›
J. Med. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
The Role of Allylic Strain for Conformational Control in Medicinal Chemistry
Journal of Medicinal Chemistry ( IF 6.8 ) Pub Date : 2023-06-07 , DOI: 10.1021/acs.jmedchem.3c00446 Hongtao Zhao 1 , Jonas Brånalt 2 , Matthew Perry 1 , Christian Tyrchan 1
Journal of Medicinal Chemistry ( IF 6.8 ) Pub Date : 2023-06-07 , DOI: 10.1021/acs.jmedchem.3c00446 Hongtao Zhao 1 , Jonas Brånalt 2 , Matthew Perry 1 , Christian Tyrchan 1
Affiliation
|
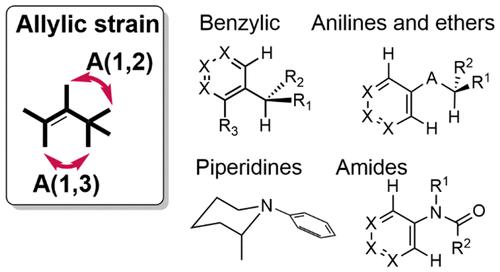
It is axiomatic in medicinal chemistry that optimization of the potency of a small molecule at a macromolecular target requires complementarity between the ligand and target. In order to minimize the conformational penalty on binding, both enthalpically and entropically, it is therefore preferred to have the ligand preorganized in the bound conformation. In this Perspective, we highlight the role of allylic strain in controlling conformational preferences. Allylic strain was originally described for carbon-based allylic systems, but the same principles apply to other types of structure with sp2 or pseudo-sp2 arrangements. These systems include benzylic (including heteroaryl methyl) positions, amides, N-aryl groups, aryl ethers, and nucleotides. We have derived torsion profiles from small molecule X-ray structures for these systems. Through multiple examples, we show how these effects have been applied in drug discovery and how they can be used prospectively to influence conformation in the design process.
中文翻译:

烯丙基应变在药物化学构象控制中的作用
药物化学中不言而喻的是,优化小分子对大分子靶点的效力需要配体和靶点之间的互补性。为了使结合的构象损失最小化,无论是热函还是熵,因此优选使配体预先组织成结合构象。在这个视角中,我们强调了烯丙基应变在控制构象偏好中的作用。烯丙基应变最初是针对碳基烯丙基系统描述的,但相同的原理也适用于具有 sp 2或伪 sp 2排列的其他类型的结构。这些系统包括苄基(包括杂芳基甲基)位置、酰胺、N-芳基、芳基醚和核苷酸。我们从这些系统的小分子 X 射线结构中得出了扭转曲线。通过多个例子,我们展示了如何将这些效应应用于药物发现,以及如何前瞻性地使用它们来影响设计过程中的构象。
更新日期:2023-06-07
中文翻译:

烯丙基应变在药物化学构象控制中的作用
药物化学中不言而喻的是,优化小分子对大分子靶点的效力需要配体和靶点之间的互补性。为了使结合的构象损失最小化,无论是热函还是熵,因此优选使配体预先组织成结合构象。在这个视角中,我们强调了烯丙基应变在控制构象偏好中的作用。烯丙基应变最初是针对碳基烯丙基系统描述的,但相同的原理也适用于具有 sp 2或伪 sp 2排列的其他类型的结构。这些系统包括苄基(包括杂芳基甲基)位置、酰胺、N-芳基、芳基醚和核苷酸。我们从这些系统的小分子 X 射线结构中得出了扭转曲线。通过多个例子,我们展示了如何将这些效应应用于药物发现,以及如何前瞻性地使用它们来影响设计过程中的构象。































 京公网安备 11010802027423号
京公网安备 11010802027423号