当前位置:
X-MOL 学术
›
J. Agric. Food Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Design, Synthesis, and Antifungal Activities of Novel Pyrazole-4-carboxamide Derivatives Containing an Ether Group as Potential Succinate Dehydrogenase Inhibitors
Journal of Agricultural and Food Chemistry ( IF 5.7 ) Pub Date : 2023-06-07 , DOI: 10.1021/acs.jafc.3c00116 Bo Luo 1 , Yacong Zhao 1 , Jing Zhang 1 , Wei Li 1 , Mengxing Liu 1 , Miaomiao Yang 1 , Lulu Wei 1 , Yijing Liu 1 , Bingjie Wen 1 , Lailiang Qu 2
Journal of Agricultural and Food Chemistry ( IF 5.7 ) Pub Date : 2023-06-07 , DOI: 10.1021/acs.jafc.3c00116 Bo Luo 1 , Yacong Zhao 1 , Jing Zhang 1 , Wei Li 1 , Mengxing Liu 1 , Miaomiao Yang 1 , Lulu Wei 1 , Yijing Liu 1 , Bingjie Wen 1 , Lailiang Qu 2
Affiliation
|
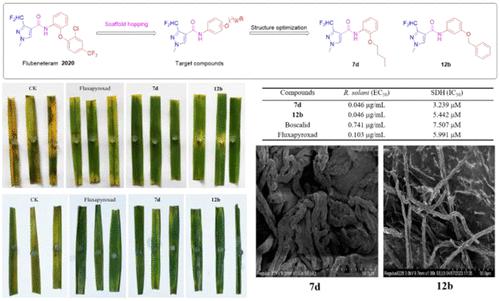
A series of novel pyrazole-4-carboxamides bearing an ether group were designed and synthesized on the basis of the structure of commercial succinate dehydrogenase inhibitor (SDHI) fungicide flubeneteram via scaffold hopping and evaluated for their antifungal activities against five fungi. The bioassay results showed that most of the target compounds exhibited excellent in vitro antifungal activity against Rhizoctonia solani and some compounds exerted remarkable antifungal activities against Sclerotinia sclerotiorum, Botrytis cinerea, Fusarium graminearum, and Alternaria alternate. Particularly, compounds 7d and 12b displayed outstanding antifungal activity against R. solani, with an EC50 value of 0.046 μg/mL, far superior to that of boscalid (EC50 = 0.741 μg/mL) and fluxapyroxad (EC50 = 0.103 μg/mL). Meanwhile, compound 12b also presented a broader fungicidal spectrum than other compounds. Moreover, in vivo anti-R. solani results showed that compounds 7d and 12b could significantly inhibit the growth of R. solani in rice leaves with excellent protective and curative efficacies. In addition, the results of the succinate dehydrogenase (SDH) enzymatic inhibition assay showed that compound 7d generated significant SDH inhibition, with an IC50 value of 3.293 μM, which was about 2 times better than that of boscalid (IC50 = 7.507 μM) and fluxapyroxad (IC50 = 5.991 μM). Furthermore, scanning electron microscopy (SEM) analysis indicated that compounds 7d and 12b significantly destroyed the typical structure and morphology of R. solani hyphae. The molecular docking study revealed that compounds 7d and 12b could embed into the binding pocket of SDH and form hydrogen bond interactions with TRP173 and TRY58 at the activity site of SDH, which was in line with fluxapyroxad, indicating that they had a similar mechanism of action. These results demonstrated that compounds 7d and 12b could be promising candidates of SDHI fungicides, which deserved further investigation.
中文翻译:

作为潜在琥珀酸脱氢酶抑制剂的新型含醚基吡唑-4-甲酰胺衍生物的设计、合成和抗真菌活性
以市售琥珀酸脱氢酶抑制剂(SDHI)杀菌剂氟苯特仑的结构为基础,通过支架跳跃设计合成了一系列带有醚基的新型吡唑-4-甲酰胺,并评价了其对五种真菌的抗真菌活性。生物测定结果表明,大部分目标化合物对立枯丝核菌具有良好的体外抗真菌活性,部分化合物对核盘菌、灰葡萄孢、禾谷镰刀菌、链格孢菌具有显着的抗真菌活性。特别地,化合物7d和12b对立枯丝核菌表现出优异的抗真菌活性,EC 50值为0.046 μg/mL,远优于啶酰菌胺(EC 50 = 0.741 μg/mL)和fluxapyroxad(EC 50 = 0.103 μg/mL)。同时,化合物12b也比其他化合物具有更广泛的杀菌谱。此外,体内抗立枯病菌结果表明,化合物7d和12b能够显着抑制水稻叶片中立枯病菌的生长,具有良好的保护和治疗作用。此外,琥珀酸脱氢酶(SDH)酶抑制实验结果表明,化合物7d产生显着的SDH抑制,IC 50值为3.293 μM,比啶酰菌胺(IC 50 = 7.507 μM)和fluxapyroxad(IC 50 = 5.991 μM)好约2倍。此外,扫描电子显微镜(SEM)分析表明,化合物7d和12b显着破坏了茄病菌菌丝的典型结构和形态。分子对接研究表明化合物7d和12b可以嵌入SDH的结合口袋并与SDH活性位点的TRP173和TRY58形成氢键相互作用,这与fluxapyroxad一致,表明它们具有相似的作用机制。这些结果表明化合物7d和12b可能是 SDHI 杀菌剂的有前途的候选者,值得进一步研究。
更新日期:2023-06-07
中文翻译:

作为潜在琥珀酸脱氢酶抑制剂的新型含醚基吡唑-4-甲酰胺衍生物的设计、合成和抗真菌活性
以市售琥珀酸脱氢酶抑制剂(SDHI)杀菌剂氟苯特仑的结构为基础,通过支架跳跃设计合成了一系列带有醚基的新型吡唑-4-甲酰胺,并评价了其对五种真菌的抗真菌活性。生物测定结果表明,大部分目标化合物对立枯丝核菌具有良好的体外抗真菌活性,部分化合物对核盘菌、灰葡萄孢、禾谷镰刀菌、链格孢菌具有显着的抗真菌活性。特别地,化合物7d和12b对立枯丝核菌表现出优异的抗真菌活性,EC 50值为0.046 μg/mL,远优于啶酰菌胺(EC 50 = 0.741 μg/mL)和fluxapyroxad(EC 50 = 0.103 μg/mL)。同时,化合物12b也比其他化合物具有更广泛的杀菌谱。此外,体内抗立枯病菌结果表明,化合物7d和12b能够显着抑制水稻叶片中立枯病菌的生长,具有良好的保护和治疗作用。此外,琥珀酸脱氢酶(SDH)酶抑制实验结果表明,化合物7d产生显着的SDH抑制,IC 50值为3.293 μM,比啶酰菌胺(IC 50 = 7.507 μM)和fluxapyroxad(IC 50 = 5.991 μM)好约2倍。此外,扫描电子显微镜(SEM)分析表明,化合物7d和12b显着破坏了茄病菌菌丝的典型结构和形态。分子对接研究表明化合物7d和12b可以嵌入SDH的结合口袋并与SDH活性位点的TRP173和TRY58形成氢键相互作用,这与fluxapyroxad一致,表明它们具有相似的作用机制。这些结果表明化合物7d和12b可能是 SDHI 杀菌剂的有前途的候选者,值得进一步研究。















































 京公网安备 11010802027423号
京公网安备 11010802027423号