当前位置:
X-MOL 学术
›
Org. Biomol. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Metal- and additive-free radical-triggered nitration/cyclization to construct indolo[2,1-α]isoquinoline and benzimidazo[2,1-a]isoquinolin-6(5H)-one derivatives using t-BuONO as nitro reagents
Organic & Biomolecular Chemistry ( IF 2.9 ) Pub Date : 2023-06-07 , DOI: 10.1039/d3ob00630a Yucai Tang 1 , Yiting Yang 1 , Qian Zhou 1 , Jinglin Duan 1 , Biyu Yang 1 , Changyuan Du 1 , Yupeng He 1
Organic & Biomolecular Chemistry ( IF 2.9 ) Pub Date : 2023-06-07 , DOI: 10.1039/d3ob00630a Yucai Tang 1 , Yiting Yang 1 , Qian Zhou 1 , Jinglin Duan 1 , Biyu Yang 1 , Changyuan Du 1 , Yupeng He 1
Affiliation
|
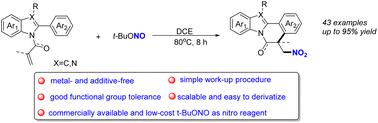
An efficient metal- and additive-free nitro radical-triggered addition/cyclization of 2-aryl-N-acryloyl indoles/2-arylbenzimidazoles for the synthesis of nitro-substituted indolo[2,1-α]isoquinoline and benzimidazo[2,1-a]isoquinolin-6(5H)-one derivatives has been developed. The commercially available and low-cost t-BuONO was used as a nitro reagent. Due to mild reaction conditions, a variety of functional groups could be tolerated to give the corresponding products in moderate to good yields. Moreover, this nitration process could be scaled-up and the nitro group could be readily converted into the amino group, which may find applications in synthetic and medicinal chemistry.
中文翻译:

使用 t-BuONO 作为硝基试剂,金属和添加剂自由基触发硝化/环化构建吲哚并[2,1-α]异喹啉和苯并咪唑并[2,1-a]异喹啉-6(5H)-酮衍生物
一种有效的无金属和添加剂的硝基引发的2-芳基-N-丙烯酰吲哚/2-芳基苯并咪唑的加成/环化反应,用于合成硝基取代的吲哚并[2,1- α ]异喹啉和苯并咪唑[2,1 -已开发出a ]isoquinolin-6(5 H )-one衍生物。使用市售且成本低廉的t -BuONO作为硝基试剂。由于反应条件温和,可以耐受多种官能团,以中等至良好的收率得到相应的产物。此外,这种硝化过程可以放大,硝基可以很容易地转化为氨基,这可能在合成和药物化学中得到应用。
更新日期:2023-06-07
中文翻译:

使用 t-BuONO 作为硝基试剂,金属和添加剂自由基触发硝化/环化构建吲哚并[2,1-α]异喹啉和苯并咪唑并[2,1-a]异喹啉-6(5H)-酮衍生物
一种有效的无金属和添加剂的硝基引发的2-芳基-N-丙烯酰吲哚/2-芳基苯并咪唑的加成/环化反应,用于合成硝基取代的吲哚并[2,1- α ]异喹啉和苯并咪唑[2,1 -已开发出a ]isoquinolin-6(5 H )-one衍生物。使用市售且成本低廉的t -BuONO作为硝基试剂。由于反应条件温和,可以耐受多种官能团,以中等至良好的收率得到相应的产物。此外,这种硝化过程可以放大,硝基可以很容易地转化为氨基,这可能在合成和药物化学中得到应用。































 京公网安备 11010802027423号
京公网安备 11010802027423号