Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Catalytic Oxidation Mechanism of Toluene over CexMn1–xO2: The Role of Oxygen Vacancies in Adsorption and Activation of Toluene
Langmuir ( IF 3.7 ) Pub Date : 2023-06-07 , DOI: 10.1021/acs.langmuir.3c00795
Wenshuo Zhou 1 , Heping Li 1 , Boying Song 1 , Wei Ma 1 , Zhikun Liu 1 , Zongcheng Wang 1 , Zhongjun Xu 2 , Liwei Meng 3 , Yafei Wang 3 , Xiaoxiao Qin 4 , Changbin Zhang 4 , Qiong Tang 5 , Xiaolei Bao 6 , Kuo Liu 7 , Hui Li 1 , Yongchun Liu 1
Langmuir ( IF 3.7 ) Pub Date : 2023-06-07 , DOI: 10.1021/acs.langmuir.3c00795
Wenshuo Zhou 1 , Heping Li 1 , Boying Song 1 , Wei Ma 1 , Zhikun Liu 1 , Zongcheng Wang 1 , Zhongjun Xu 2 , Liwei Meng 3 , Yafei Wang 3 , Xiaoxiao Qin 4 , Changbin Zhang 4 , Qiong Tang 5 , Xiaolei Bao 6 , Kuo Liu 7 , Hui Li 1 , Yongchun Liu 1
Affiliation
|
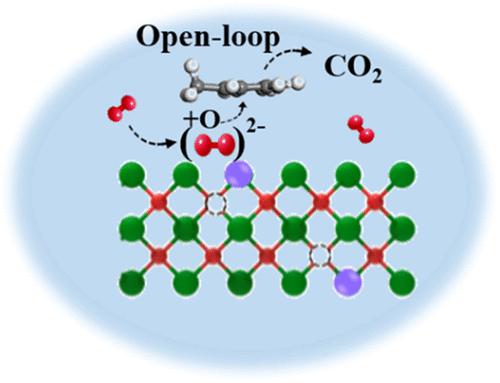
Catalytic oxidation has been extensively studied as a promising technology for the removal of toluene from industrial waste gases and indoor air. However, the debate regarding the oxidation mechanism is far from resolved. CexMn1–xO2 catalysts with different mixing ratios are prepared by the sol–gel method and found to exhibit better catalytic activities for toluene oxidation than a single oxide. Characterizations and theoretical calculations reveal that the doped Mn increases the number of oxygen vacancies and the ability of oxygen vacancies to activate aromatic rings, which promotes the rate-determining step of toluene oxidation, i.e., ring-opening reactions. The oxidation products detected by in situ diffuse reflectance infrared Fourier transform spectroscopy (DRIFTS) and Vocus proton transfer reaction mass spectrometry (Vocus-PTR-MS) show that the doped Mn significantly improves the ring-opening efficiency and subsequently yields more short-chain products, such as pyruvic acid and acetic acid. A comprehensive oxidation pathway of toluene is refined in this work.
中文翻译:

CexMn1-xO2 上的甲苯催化氧化机理:氧空位在甲苯吸附和活化中的作用
催化氧化作为一种有前途的从工业废气和室内空气中去除甲苯的技术已被广泛研究。然而,关于氧化机制的争论还远未解决。采用溶胶-凝胶法制备了不同混合比例的Ce x Mn 1– x O 2催化剂,发现其对甲苯氧化的催化活性优于单一氧化物。表征和理论计算表明,Mn的掺杂增加了氧空位的数量和氧空位激活芳环的能力,从而促进了甲苯氧化的决速步骤,即开环反应。原位检测氧化产物漫反射红外傅里叶变换光谱(DRIFTS)和Vocus质子转移反应质谱(Vocus-PTR-MS)表明,掺杂的Mn显着提高了开环效率,从而产生更多的短链产物,例如丙酮酸和乙酸酸。这项工作完善了甲苯的综合氧化途径。
更新日期:2023-06-07
中文翻译:

CexMn1-xO2 上的甲苯催化氧化机理:氧空位在甲苯吸附和活化中的作用
催化氧化作为一种有前途的从工业废气和室内空气中去除甲苯的技术已被广泛研究。然而,关于氧化机制的争论还远未解决。采用溶胶-凝胶法制备了不同混合比例的Ce x Mn 1– x O 2催化剂,发现其对甲苯氧化的催化活性优于单一氧化物。表征和理论计算表明,Mn的掺杂增加了氧空位的数量和氧空位激活芳环的能力,从而促进了甲苯氧化的决速步骤,即开环反应。原位检测氧化产物漫反射红外傅里叶变换光谱(DRIFTS)和Vocus质子转移反应质谱(Vocus-PTR-MS)表明,掺杂的Mn显着提高了开环效率,从而产生更多的短链产物,例如丙酮酸和乙酸酸。这项工作完善了甲苯的综合氧化途径。

































 京公网安备 11010802027423号
京公网安备 11010802027423号