Synthesis ( IF 2.2 ) Pub Date : 2023-06-06 , DOI: 10.1055/a-2039-5424 Aaron D. G. Campbell 1 , Roly J. Armstrong 1
|
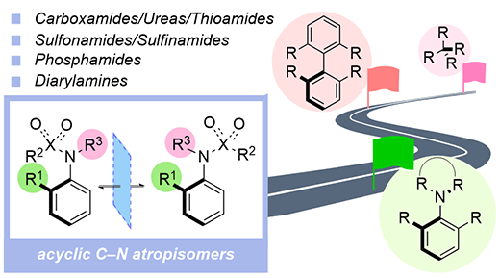
Atropisomeric molecules are a privileged class of stereogenic material that have important applications in catalysis, materials science and medicines. To date, the majority of work has been focused upon biaryl and heterobiaryl scaffolds involving restricted rotation between a pair of cyclic fragments, but C–N atropisomeric molecules based upon amines and amides, where the nitrogen atom is not part of a ring system, are rapidly emerging as an important class of stereogenic molecules. This is the focus of this Short Review, which begins by discussing the factors which influence the configurational stability of such molecules and provides a historical background to their synthesis. This is followed by a detailed discussion of state-of-the-art catalytic asymmetric strategies that are now available to access C–Nacyclic atropisomers including carboxamides, sulfonamides, sulfinamides, phosphamides and diarylamines. A variety of different synthetic approaches are discussed, including kinetic resolution/desymmetrization, amination, C–H functionalization, N-functionalization, and annulation.
1 Introduction
2 Atropisomerism in Acyclic Amines and Amides
3 Synthesis Directed by a Chiral Auxiliary
4 Atropselective Synthesis
4.1 Kinetic Resolution and Desymmetrization
4.2 Electrophilic Amination
4.3 C–H Functionalization
4.4 N-Functionalization
4.5 Annulation
5 Conclusions and Outlook
中文翻译:

控制无环胺和酰胺中 C-N 阻转异构的合成策略
阻转异构分子是一类特殊的立体异构材料,在催化、材料科学和医学中具有重要应用。迄今为止,大部分工作都集中在联芳基和杂联芳基支架上,涉及一对环状片段之间的受限旋转,但是基于胺和酰胺的 C-N 阻转异构分子,其中氮原子不是环系统的一部分,是迅速成为一类重要的立体异构分子。这是本篇短评的重点,它首先讨论影响此类分子构型稳定性的因素,并提供其合成的历史背景。接下来是对最先进的催化不对称策略的详细讨论,这些策略现在可用于访问 C-N无环阻转异构体包括甲酰胺、磺酰胺、亚磺酰胺、磷酰胺和二芳基胺。讨论了各种不同的合成方法,包括动力学拆分/去对称化、胺化、C-H 功能化、N-功能化和环化。
1 简介
2 无环胺和酰胺的阻转异构现象
3 手性助剂指导的合成
4 选择性阻滞合成
4.1 动力学拆分和去对称化
4.2 亲电胺化
4.3 C-H功能化
4.4 N-功能化
4.5 年化
5 结论与展望































 京公网安备 11010802027423号
京公网安备 11010802027423号