当前位置:
X-MOL 学术
›
Chem. Bio. Drug Des.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Synthetic approaches and applications of an underprivileged 1,2,5-oxadiazole moiety: A review
Chemical Biology & Drug Design ( IF 3.2 ) Pub Date : 2023-06-05 , DOI: 10.1111/cbdd.14276 Greesh Kumar 1 , Rajnish Kumar 1 , Avijit Mazumder 1 , Salahuddin 1 , Upendra Kumar 1
Chemical Biology & Drug Design ( IF 3.2 ) Pub Date : 2023-06-05 , DOI: 10.1111/cbdd.14276 Greesh Kumar 1 , Rajnish Kumar 1 , Avijit Mazumder 1 , Salahuddin 1 , Upendra Kumar 1
Affiliation
|
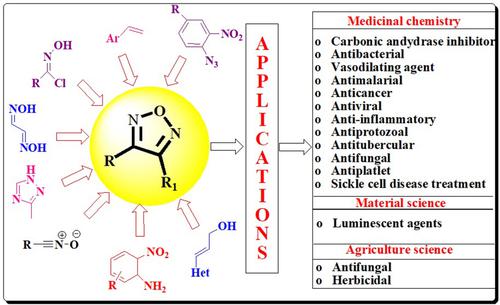
1,2,5-oxadiazole belongs to five-membered heterocyclic compounds with two nitrogen and one oxygen atom. In comparison with other heterocyclic moieties, 1,2,5-oxadiazoles moiety is considered as underprivileged as it attracted little attention of the researchers although lot of scopes and possible applications in medicinal, material and agriculture science. 1,2,5-oxadiazole and its derivatives have been reported as good pharmacophores as carbonic anhydrase inhibitors, antibacterial, vasodilating agents, antimalarial, anticancer, etc. In the presented manuscript, we reviewed granted patents and different synthetic strategies which have been reported for the synthesis of 1,2,5-oxadiazoles such as cycloaddition, dimerization, cyclodehydration, condensation, thermolysis, nitration, oxidation and ring-conversion. These synthetic methods have also been analysed for their merits and demerits. The manuscript also highlighted various applications of 1,2,5-oxadiazole and its derivatives. We hope that researchers across the scientific streams will be benefitted from the presented review articles for designing their work related to 1,2,5-oxadiazoles.
中文翻译:

1,2,5-恶二唑部分的合成方法和应用:综述
1,2,5-恶二唑属于具有两个氮和一个氧原子的五元杂环化合物。与其他杂环基团相比,1,2,5-恶二唑基团被认为是弱势的,尽管它在医学、材料和农业科学中具有广泛的范围和可能的应用,但很少引起研究人员的关注。1,2,5-恶二唑及其衍生物已被报道为良好的药效团,如碳酸酐酶抑制剂、抗菌剂、血管舒张剂、抗疟药、抗癌药等。在本文中,我们回顾了已报道的授权专利和不同的合成策略。 1,2,5-恶二唑的合成,如环加成、二聚、环化脱水、缩合、热解、硝化、氧化和环化。还分析了这些合成方法的优缺点。该手稿还强调了 1,2,5-恶二唑及其衍生物的各种应用。我们希望各科学领域的研究人员能够从所提交的综述文章中受益,以设计与 1,2,5-恶二唑相关的工作。
更新日期:2023-06-05
中文翻译:

1,2,5-恶二唑部分的合成方法和应用:综述
1,2,5-恶二唑属于具有两个氮和一个氧原子的五元杂环化合物。与其他杂环基团相比,1,2,5-恶二唑基团被认为是弱势的,尽管它在医学、材料和农业科学中具有广泛的范围和可能的应用,但很少引起研究人员的关注。1,2,5-恶二唑及其衍生物已被报道为良好的药效团,如碳酸酐酶抑制剂、抗菌剂、血管舒张剂、抗疟药、抗癌药等。在本文中,我们回顾了已报道的授权专利和不同的合成策略。 1,2,5-恶二唑的合成,如环加成、二聚、环化脱水、缩合、热解、硝化、氧化和环化。还分析了这些合成方法的优缺点。该手稿还强调了 1,2,5-恶二唑及其衍生物的各种应用。我们希望各科学领域的研究人员能够从所提交的综述文章中受益,以设计与 1,2,5-恶二唑相关的工作。








































 京公网安备 11010802027423号
京公网安备 11010802027423号