当前位置:
X-MOL 学术
›
Arch. Pharm.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Targeting Mycobacterium tuberculosis: Synthesis, in vitro and in silico evaluation of novel N1-(benzo[d]oxazol-2-yl)-N4-arylidine compounds
Archiv der Pharmazie ( IF 4.3 ) Pub Date : 2023-06-06 , DOI: 10.1002/ardp.202300187 Amira G Zawal 1 , Marwa M Abdel-Aziz 2 , Abdalla A El-Shanawani 1 , Lobna M Abdel-Aziz 1 , Mohamed Taha 3 , Claire Simons 4 , Samar S Elbaramawi 1
Archiv der Pharmazie ( IF 4.3 ) Pub Date : 2023-06-06 , DOI: 10.1002/ardp.202300187 Amira G Zawal 1 , Marwa M Abdel-Aziz 2 , Abdalla A El-Shanawani 1 , Lobna M Abdel-Aziz 1 , Mohamed Taha 3 , Claire Simons 4 , Samar S Elbaramawi 1
Affiliation
|
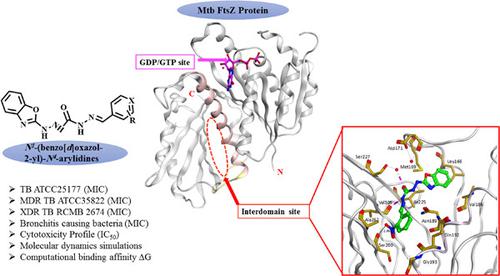
The development of novel antimycobacterial agents is an urgent challenge to eradicate the increasing emergence and rapid spread of multidrug-resistant strains. Filamentous temperature-sensitive protein Z (FtsZ) is a crucial cell division protein. Alteration of FtsZ assembly leads to cell division inhibition and cell death. To find novel antimycobacterial agents, a series of N1-(benzo[d]oxazol-2-yl)-N4-arylidine compounds 5a–o were synthesized. The activity of the compounds was evaluated against drug-sensitive, multidrug-resistant, and extensive-drug-resistant Mycobacterium tuberculosis. Compounds 5b, 5c, 5l, 5m, and 5o showed promising antimycobacterial activity with minimum inhibitory concentrations (MIC) in the range of 0.48–1.85 µg/mL and with low cytotoxicity against human nontumorigenic lung fibroblast WI-38 cells. The activity of the compounds 5b, 5c, 5l, 5m, and 5o was evaluated against bronchitis causing-bacteria. They exhibited good activity against Streptococcus pneumoniae, Klebsiella pneumoniae, Mycoplasma pneumonia, and Bordetella pertussis. Molecular dynamics simulations of Mtb FtsZ protein-ligand complexes identified the interdomain site as the binding site and key interactions. ADME prediction indicated that the synthesized compounds have drug-likeness. The density function theory studies of 5c, 5l, and 5n were performed to investigate E/Z isomerization. Compounds 5c and 5l are present as E-isomers and 5n as an E/Z mixture. Our experimental outcomes provide an auspicious lead for the design of more selective and potent antimycobacterial drugs.
中文翻译:

靶向结核分枝杆菌:新型 N1-(苯并[d]恶唑-2-基)-N4-亚芳基化合物的合成、体外和计算机评估
新型抗分枝杆菌药物的开发是消除日益出现和快速传播的多重耐药菌株的紧迫挑战。丝状温度敏感蛋白 Z (FtsZ) 是一种重要的细胞分裂蛋白。FtsZ 组装的改变会导致细胞分裂抑制和细胞死亡。为了寻找新型抗分枝杆菌药物,合成了一系列N 1 -(苯并[ d ]恶唑-2-基) -N 4 -亚芳基化合物5a–o 。评估了这些化合物针对药物敏感、多重耐药和广泛耐药结核分枝杆菌的活性。化合物5b、5c、5l、5m和5o显示出良好的抗分枝杆菌活性,最低抑菌浓度 (MIC) 在 0.48–1.85 µg/mL 范围内,并且对人非致瘤肺成纤维细胞 WI-38 细胞具有低细胞毒性。评价化合物5b、5c、5l、5m和5o针对引起支气管炎的细菌的活性。它们对肺炎链球菌、肺炎克雷伯菌、肺炎支原体和百日咳博德特氏菌表现出良好的活性。Mtb FtsZ 蛋白-配体复合物的分子动力学模拟确定了域间位点作为结合位点和关键相互作用。ADME预测表明合成的化合物具有药物相似性。进行5c、5l和5n的密度函数理论研究以研究E/Z异构化。化合物5c和5l以E异构体形式存在,5n以E/Z混合物形式存在。我们的实验结果为设计更具选择性和更有效的抗分枝杆菌药物提供了良好的指导。
更新日期:2023-06-07
中文翻译:

靶向结核分枝杆菌:新型 N1-(苯并[d]恶唑-2-基)-N4-亚芳基化合物的合成、体外和计算机评估
新型抗分枝杆菌药物的开发是消除日益出现和快速传播的多重耐药菌株的紧迫挑战。丝状温度敏感蛋白 Z (FtsZ) 是一种重要的细胞分裂蛋白。FtsZ 组装的改变会导致细胞分裂抑制和细胞死亡。为了寻找新型抗分枝杆菌药物,合成了一系列N 1 -(苯并[ d ]恶唑-2-基) -N 4 -亚芳基化合物5a–o 。评估了这些化合物针对药物敏感、多重耐药和广泛耐药结核分枝杆菌的活性。化合物5b、5c、5l、5m和5o显示出良好的抗分枝杆菌活性,最低抑菌浓度 (MIC) 在 0.48–1.85 µg/mL 范围内,并且对人非致瘤肺成纤维细胞 WI-38 细胞具有低细胞毒性。评价化合物5b、5c、5l、5m和5o针对引起支气管炎的细菌的活性。它们对肺炎链球菌、肺炎克雷伯菌、肺炎支原体和百日咳博德特氏菌表现出良好的活性。Mtb FtsZ 蛋白-配体复合物的分子动力学模拟确定了域间位点作为结合位点和关键相互作用。ADME预测表明合成的化合物具有药物相似性。进行5c、5l和5n的密度函数理论研究以研究E/Z异构化。化合物5c和5l以E异构体形式存在,5n以E/Z混合物形式存在。我们的实验结果为设计更具选择性和更有效的抗分枝杆菌药物提供了良好的指导。








































 京公网安备 11010802027423号
京公网安备 11010802027423号