当前位置:
X-MOL 学术
›
ACS Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Dynamically Formed Active Sites on Liquid Boron Oxide for Selective Oxidative Dehydrogenation of Propane
ACS Catalysis ( IF 11.3 ) Pub Date : 2023-06-05 , DOI: 10.1021/acscatal.3c01759 Jinshu Tian 1, 2, 3 , Gregory Collinge 1 , Simuck F. Yuk 1, 4 , Jindong Lin 3 , Vassiliki-Alexandra Glezakou 1 , Mal-Soon Lee 1 , Yong Wang 1, 5 , Roger Rousseau 1
ACS Catalysis ( IF 11.3 ) Pub Date : 2023-06-05 , DOI: 10.1021/acscatal.3c01759 Jinshu Tian 1, 2, 3 , Gregory Collinge 1 , Simuck F. Yuk 1, 4 , Jindong Lin 3 , Vassiliki-Alexandra Glezakou 1 , Mal-Soon Lee 1 , Yong Wang 1, 5 , Roger Rousseau 1
Affiliation
|
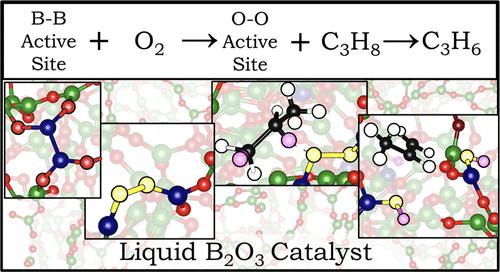
Boron oxide-based catalysts have been shown to be both active and selective for driving the oxidative dehydrogenation of propane (ODHP) without the use of metal promoters. However, this reaction occurs at temperatures where boron oxide melts, challenging experimental identification of the molecular structures within the boron oxide phase under reaction conditions and thus hindering the understanding of its active sites and reaction mechanism(s). By combining density functional theory computations, ab initio molecular dynamics simulations, in situ Raman characterization, and microkinetic modeling, we propose that dimerized di-coordinated boron sites (>B–B<)─dynamically formed in liquid boron oxide─are the active species for O2 activation under reaction conditions. The resulting peroxy-like species (>B–O–O–B<) is then responsible for propane activation but is a moderate oxidant for ODHP and thus inert to propene. These peroxy-like structures rapidly activate propane, homolytically cleaving the >B–O–O–B< bond, producing a propyl radical and a >B–O• dangling bond. These > B–O• originate from the >B–O–O–B< sites as well as the liquid B2O3 structure itself and play a critical role in the abstraction of H atoms from propane and propyl. In fact, microkinetic modeling reveals that the formation of adsorbed C3H7* radicals is the main rate-controlling step due to the highly endergonic adsorption of propane into the system. Otherwise, the only activated processes were found to be the dehydration steps that lead to water formation, which exhibit an intriguing dependence on the concentration of surface hydroxyl species. These findings provide significant insights into the ODHP mechanisms on boron-based catalysts and emphasize the importance of understanding the liquid nature of the oxide to account for its catalytic activity.
中文翻译:

液体氧化硼上动态形成的活性位点用于丙烷的选择性氧化脱氢
已证明基于氧化硼的催化剂在不使用金属促进剂的情况下对驱动丙烷氧化脱氢 (ODHP) 具有活性和选择性。然而,这种反应发生在氧化硼熔化的温度下,这对反应条件下氧化硼相内分子结构的实验鉴定具有挑战性,从而阻碍了对其活性位点和反应机制的理解。通过结合密度泛函理论计算、从头算分子动力学模拟、原位拉曼表征和微观动力学建模,我们提出二聚双配位硼位点 (>B–B<)──在液态氧化硼中动态形成──是活性物质对于 O 2在反应条件下活化。由此产生的类过氧化物 (>B–O–O–B<) 负责丙烷活化,但它是 ODHP 的适度氧化剂,因此对丙烯呈惰性。这些类似过氧化物的结构迅速激活丙烷,均裂裂解 >B–O–O–B< 键,产生丙基自由基和 >B–O • 悬空键。这些 > B–O •源自 >B–O–O–B< 位点以及液体 B 2 O 3结构本身,并且在从丙烷和丙基中提取 H 原子方面发挥关键作用。事实上,微观动力学模型表明吸附的 C 3 H 7的形成* 由于丙烷在系统中的高吸能吸附,自由基是主要的速率控制步骤。否则,发现唯一激活的过程是导致水形成的脱水步骤,这表现出对表面羟基物质浓度的有趣依赖性。这些发现为硼基催化剂的 ODHP 机制提供了重要的见解,并强调了了解氧化物的液体性质以解释其催化活性的重要性。
更新日期:2023-06-05
中文翻译:

液体氧化硼上动态形成的活性位点用于丙烷的选择性氧化脱氢
已证明基于氧化硼的催化剂在不使用金属促进剂的情况下对驱动丙烷氧化脱氢 (ODHP) 具有活性和选择性。然而,这种反应发生在氧化硼熔化的温度下,这对反应条件下氧化硼相内分子结构的实验鉴定具有挑战性,从而阻碍了对其活性位点和反应机制的理解。通过结合密度泛函理论计算、从头算分子动力学模拟、原位拉曼表征和微观动力学建模,我们提出二聚双配位硼位点 (>B–B<)──在液态氧化硼中动态形成──是活性物质对于 O 2在反应条件下活化。由此产生的类过氧化物 (>B–O–O–B<) 负责丙烷活化,但它是 ODHP 的适度氧化剂,因此对丙烯呈惰性。这些类似过氧化物的结构迅速激活丙烷,均裂裂解 >B–O–O–B< 键,产生丙基自由基和 >B–O • 悬空键。这些 > B–O •源自 >B–O–O–B< 位点以及液体 B 2 O 3结构本身,并且在从丙烷和丙基中提取 H 原子方面发挥关键作用。事实上,微观动力学模型表明吸附的 C 3 H 7的形成* 由于丙烷在系统中的高吸能吸附,自由基是主要的速率控制步骤。否则,发现唯一激活的过程是导致水形成的脱水步骤,这表现出对表面羟基物质浓度的有趣依赖性。这些发现为硼基催化剂的 ODHP 机制提供了重要的见解,并强调了了解氧化物的液体性质以解释其催化活性的重要性。































 京公网安备 11010802027423号
京公网安备 11010802027423号