当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Discovery of Itaconate-Mediated Lysine Acylation
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2023-06-04 , DOI: 10.1021/jacs.3c02332 Dongyang Liu 1 , Weidi Xiao 1 , Haoting Li 2 , Yanling Zhang 3 , Shouli Yuan 1 , Chengxi Li 1 , Suwei Dong 2 , Chu Wang 1, 3
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2023-06-04 , DOI: 10.1021/jacs.3c02332 Dongyang Liu 1 , Weidi Xiao 1 , Haoting Li 2 , Yanling Zhang 3 , Shouli Yuan 1 , Chengxi Li 1 , Suwei Dong 2 , Chu Wang 1, 3
Affiliation
|
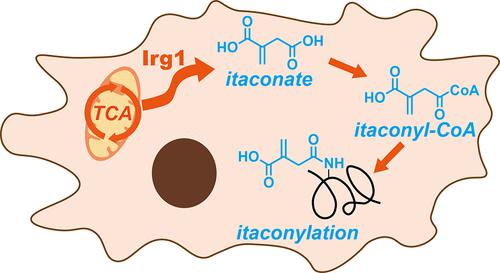
Itaconate is an important antimicrobial and immunoregulatory metabolite involved in host–pathogen interactions. A key mechanistic action of itaconate is through the covalent modification of cysteine residues via Michael addition, resulting in “itaconation”. However, it is unclear whether itaconate has other regulatory mechanisms. In this work, we discovered a novel type of post-translational modification by promiscuous antibody enrichment and data analysis with the open-search strategy and further confirmed it as the lysine “itaconylation”. We showed that itaconylation and its precursor metabolite itaconyl-CoA undergo significant upregulation upon lipopolysaccharides (LPS) stimulation in RAW264.7 macrophages. Quantitative proteomics identified itaconylation sites in multiple functional proteins, including glycolytic enzymes and histones, some of which were confirmed by synthetic peptide standards. The discovery of lysine itaconylation opens up new areas for studying how itaconate participates in immunoregulation via protein post-translational modification.
中文翻译:

发现衣康酸介导的赖氨酸酰化
衣康酸盐是一种重要的抗菌和免疫调节代谢物,参与宿主-病原体相互作用。衣康酸盐的一个关键机制作用是通过迈克尔加成对半胱氨酸残基进行共价修饰,从而导致“衣康化”。然而,尚不清楚衣康酸盐是否具有其他调节机制。在这项工作中,我们通过开放搜索策略的混杂抗体富集和数据分析发现了一种新型的翻译后修饰,并进一步确认其为赖氨酸“衣康酰化”。我们表明衣康酰化及其前体代谢物衣康酰辅酶 A 在 RAW264.7 巨噬细胞中对脂多糖 (LPS) 刺激进行显着上调。定量蛋白质组学鉴定了多种功能蛋白中的衣康基化位点,包括糖酵解酶和组蛋白,其中一些已通过合成肽标准得到证实。赖氨酸衣康酰化的发现为研究衣康酸如何通过蛋白质翻译后修饰参与免疫调节开辟了新领域。
更新日期:2023-06-04
中文翻译:

发现衣康酸介导的赖氨酸酰化
衣康酸盐是一种重要的抗菌和免疫调节代谢物,参与宿主-病原体相互作用。衣康酸盐的一个关键机制作用是通过迈克尔加成对半胱氨酸残基进行共价修饰,从而导致“衣康化”。然而,尚不清楚衣康酸盐是否具有其他调节机制。在这项工作中,我们通过开放搜索策略的混杂抗体富集和数据分析发现了一种新型的翻译后修饰,并进一步确认其为赖氨酸“衣康酰化”。我们表明衣康酰化及其前体代谢物衣康酰辅酶 A 在 RAW264.7 巨噬细胞中对脂多糖 (LPS) 刺激进行显着上调。定量蛋白质组学鉴定了多种功能蛋白中的衣康基化位点,包括糖酵解酶和组蛋白,其中一些已通过合成肽标准得到证实。赖氨酸衣康酰化的发现为研究衣康酸如何通过蛋白质翻译后修饰参与免疫调节开辟了新领域。













































 京公网安备 11010802027423号
京公网安备 11010802027423号