当前位置:
X-MOL 学术
›
Ind. Eng. Chem. Res.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Liquid–Liquid Equilibrium Measurements and Interaction Explorations for Separation of Dimethyl Carbonate and Isopropyl Alcohol Using ChCl-Based Deep Eutectic Solvents
Industrial & Engineering Chemistry Research ( IF 3.8 ) Pub Date : 2023-06-05 , DOI: 10.1021/acs.iecr.3c01045 Chao Sun 1, 2 , Jiafu Xing 1 , Yajuan Qu 1 , Yanli Zhang 1 , Peizhe Cui 1 , Yinglong Wang 1 , Jun Gao 3
Industrial & Engineering Chemistry Research ( IF 3.8 ) Pub Date : 2023-06-05 , DOI: 10.1021/acs.iecr.3c01045 Chao Sun 1, 2 , Jiafu Xing 1 , Yajuan Qu 1 , Yanli Zhang 1 , Peizhe Cui 1 , Yinglong Wang 1 , Jun Gao 3
Affiliation
|
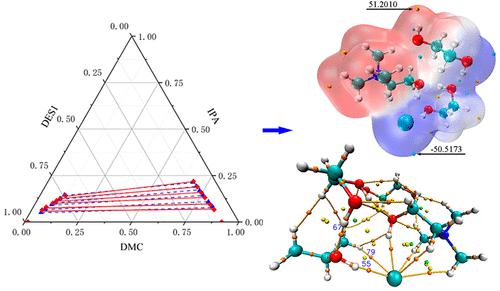
Deep eutectic solvents (DESs) were adopted to separate the azeotropes of dimethyl carbonate (DMC) and isopropyl alcohol (IPA). Liquid–liquid equilibrium (LLE) data plays a crucial role in designing the separation process. Three appropriate DESs (choline chloride (ChCl)-Urea, ChCl-Glycerol (Gly), ChCl-Ethylene glycol (EG)) were selected using the COMSO model. Then, the LLE data of DMC-IPA-(ChCl-Urea), DMC-IPA-(ChCl-EG), and DMC-IPA-(ChCl-Gly) were obtained through experiments at 298.15 K under 101.32 kPa, the binary interaction parameters were obtained by regressing the LLE data by the nonrandom two-liquid (NRTL) model, and the GUI-Matlab was adopted to verify the precision of the NRTL interaction parameters and the experimental data. Finally, the mechanism of formation of the combined structures (DES-IPA/DMC) was explored through ESP. To further explore the extraction capacities of the selected DESs, the extraction mechanism of three DESs was revealed. The interactions between DESs and IPA/DMC were explored through penetration distance and AIM analysis; the calculation results showed that (ChCl-EG) has the best extraction capacity, which is consistent with the experimental results. The results of experimental and theoretical calculations showed that the DES could effectively separate DMC and IPA.
中文翻译:

使用 ChCl 基低共熔溶剂分离碳酸二甲酯和异丙醇的液-液平衡测量和相互作用探索
采用低共熔溶剂 (DES) 分离碳酸二甲酯 (DMC) 和异丙醇 (IPA) 的共沸物。液液平衡 (LLE) 数据在设计分离过程中起着至关重要的作用。使用 COMSO 模型选择了三种合适的 DES(氯化胆碱 (ChCl)-尿素、ChCl-甘油 (Gly)、ChCl-乙二醇 (EG))。然后,通过在 298.15 K 和 101.32 kPa 下的实验获得 DMC-IPA-(ChCl-Urea)、DMC-IPA-(ChCl-EG) 和 DMC-IPA-(ChCl-Gly) 的 LLE 数据,二元相互作用通过非随机双液(NRTL)模型对LLE数据进行回归得到参数,并采用GUI-Matlab验证NRTL相互作用参数与实验数据的精度。最后,通过ESP探索了组合结构(DES-IPA/DMC)的形成机制。为了进一步探索所选 DES 的提取能力,揭示了三种 DES 的提取机制。通过穿透距离和 AIM 分析探讨了 DES 与 IPA/DMC 之间的相互作用;计算结果表明(ChCl-EG)具有最好的提取能力,与实验结果一致。实验和理论计算结果表明,DES可以有效分离DMC和IPA。
更新日期:2023-06-05
中文翻译:

使用 ChCl 基低共熔溶剂分离碳酸二甲酯和异丙醇的液-液平衡测量和相互作用探索
采用低共熔溶剂 (DES) 分离碳酸二甲酯 (DMC) 和异丙醇 (IPA) 的共沸物。液液平衡 (LLE) 数据在设计分离过程中起着至关重要的作用。使用 COMSO 模型选择了三种合适的 DES(氯化胆碱 (ChCl)-尿素、ChCl-甘油 (Gly)、ChCl-乙二醇 (EG))。然后,通过在 298.15 K 和 101.32 kPa 下的实验获得 DMC-IPA-(ChCl-Urea)、DMC-IPA-(ChCl-EG) 和 DMC-IPA-(ChCl-Gly) 的 LLE 数据,二元相互作用通过非随机双液(NRTL)模型对LLE数据进行回归得到参数,并采用GUI-Matlab验证NRTL相互作用参数与实验数据的精度。最后,通过ESP探索了组合结构(DES-IPA/DMC)的形成机制。为了进一步探索所选 DES 的提取能力,揭示了三种 DES 的提取机制。通过穿透距离和 AIM 分析探讨了 DES 与 IPA/DMC 之间的相互作用;计算结果表明(ChCl-EG)具有最好的提取能力,与实验结果一致。实验和理论计算结果表明,DES可以有效分离DMC和IPA。





















































 京公网安备 11010802027423号
京公网安备 11010802027423号