Nano Research ( IF 9.5 ) Pub Date : 2023-06-05 , DOI: 10.1007/s12274-023-5824-6
Yumei Liu , Tiantian Wu , Hongfei Cheng , Jiawen Wu , Xiaodong Guo , Hong Jin Fan
|
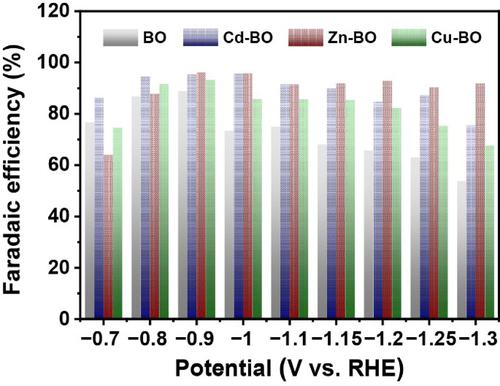
Formic acid is considered one of the most economically viable products for electrocatalytic CO2 reduction reaction (CO2RR). However, developing highly active and selective electrocatalysts for effective CO2 conversion remains a grand challenge. Herein, we report that structural modulation of the bismuth oxide nanosheet via Zn2+ cooperation has a profound positive effect on exposure of the active plane, thereby contributing to high electrocatalytic CO2RR performance. The obtained Zn-Bi2O3 catalyst demonstrates superior selectivity towards formate generation in a wide potential range; a high Faradaic efficiency of 95% and a desirable partial current density of around 20 mA·cm−2 are obtained at −0.9 V (vs. reversible hydrogen electrode (RHE)). As proposed by density functional theory calculations, Zn substitution is the most energetically feasible for forming and stabilizing the key OCHO* intermediate among the used metal ions. Moreover, the more negative adsorption energy of OCHO* and the relatively low energy barrier for the desorption of HCOOH* are responsible for the enhanced activity and selectivity.
中文翻译:

通过 Zn 取代 Bi2O3 纳米片的活性平面调制以有效电催化 CO2 还原为甲酸
甲酸被认为是用于电催化 CO 2还原反应 (CO 2 RR) 的最经济可行的产品之一。然而,开发用于有效CO 2转化的高活性和选择性电催化剂仍然是一个巨大的挑战。在此,我们报道了通过 Zn 2+合作对氧化铋纳米片的结构调制对活性平面的暴露具有深远的积极影响,从而有助于提高电催化 CO 2 RR 性能。得到的Zn-Bi 2 O 3催化剂在广泛的潜在范围内对甲酸盐生成表现出优异的选择性;在-0.9 V(相对于可逆氢电极(RHE))下获得了95% 的高法拉第效率和约20 mA·cm -2的理想分电流密度。正如密度泛函理论计算所提出的,Zn 取代是在所用金属离子中形成和稳定关键 OCHO* 中间体的最具活力的可行方法。此外,OCHO* 的负吸附能和 HCOOH* 的解吸能量势垒相对较低,这也是提高活性和选择性的原因。

































 京公网安备 11010802027423号
京公网安备 11010802027423号