当前位置:
X-MOL 学术
›
J. Mol. Liq.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Extraction of C6 α-olefin against n-paraffin in coker naphtha with deep eutectic solvents: Quantum chemical calculations and experimental investigation
Journal of Molecular Liquids ( IF 5.3 ) Pub Date : 2023-06-03 , DOI: 10.1016/j.molliq.2023.122256 Fei Luo , Lihua Liu , Ximing Chen , Lanyi Sun
Journal of Molecular Liquids ( IF 5.3 ) Pub Date : 2023-06-03 , DOI: 10.1016/j.molliq.2023.122256 Fei Luo , Lihua Liu , Ximing Chen , Lanyi Sun
|
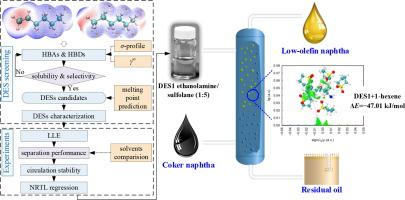
Extraction technology is often employed to separate olefin/paraffin mixtures due to the advantages of mature process, flexible operation, and large processing capacity. This work aims to investigate the effectiveness of novel deep eutectic solvents (DESs) for the extraction of 1-hexene/-hexane model coker naphtha using a combination of simulations and experiments. The results showed that the aliphatic or aromatic groups in the hydrogen bond acceptors or hydrogen bond donors have a strong affinity for 1-hexene, and the components containing hydroxyl or aromatic groups possess the ability to provide protons, which is beneficial to dissolve 1-hexene. Combining the results of the melting point predicted by the group and group-interaction contribution model, three DESs, namely ethanolamine-sulfolane 1:5 (DES1), tetrabutylammonium bromide-bis[2-(2-hydroxyethoxy)ethyl] ether 1:4 (DES2), and methyltriphenylphosphonium bromide-Triethylene glycol 1:4 (DES3), were finally prepared. Then, quantum chemical calculations were used to investigate the separation mechanism between the DESs and 1-hexene/-hexane, demonstrating single-center and multi-center hybrid weak hydrogen bonds dominated by the dispersion attraction between DESs and 1-hexene. Finally, liquid–liquid equilibrium experiments were used to examine the effects of DESs type, composition, and temperature on the separation performance, and the results showed that the DES1 has both high distribution coefficient of 1-hexene and selectivity of 1-hexene/-hexane, as well as good recycle stability. This work demonstrates that new DESs can be used as alternative solvents to traditional organic solvents and ionic liquids, and provides ideas for the separation of olefins and paraffins in coker naphtha.
中文翻译:

用低共熔溶剂萃取焦化石脑油中的 C6 α-烯烃:量子化学计算和实验研究
萃取技术具有工艺成熟、操作灵活、处理能力大等优点,常用于分离烯烃/石蜡混合物。这项工作旨在通过模拟和实验相结合的方式研究新型低共熔溶剂 (DES) 用于萃取 1-己烯/-己烷模型焦化石脑油的有效性。结果表明,氢键受体或氢键供体中的脂肪族或芳香族基团对1-己烯具有较强的亲和力,且含有羟基或芳香族基团的组分具有提供质子的能力,有利于1-己烯的溶解。 。结合基团和基团相互作用贡献模型预测的熔点结果,三种DES,即乙醇胺-环丁砜1:5(DES1)、四丁基溴化铵-双[2-(2-羟基乙氧基)乙基]醚1:4最终制备了(DES2)和甲基三苯基溴化鏻-三甘醇1:4(DES3)。然后,利用量子化学计算研究了DES和1-己烯/-己烷之间的分离机制,证明了由DES和1-己烯之间的色散吸引力主导的单中心和多中心混合弱氢键。最后,通过液-液平衡实验考察了DES的类型、组成和温度对分离性能的影响,结果表明DES1既具有高的1-己烯分配系数,又具有1-己烯/-的选择性。己烷,以及良好的循环稳定性。这项工作表明新型DES可以作为传统有机溶剂和离子液体的替代溶剂,并为焦化石脑油中烯烃和烷烃的分离提供思路。
更新日期:2023-06-03
中文翻译:

用低共熔溶剂萃取焦化石脑油中的 C6 α-烯烃:量子化学计算和实验研究
萃取技术具有工艺成熟、操作灵活、处理能力大等优点,常用于分离烯烃/石蜡混合物。这项工作旨在通过模拟和实验相结合的方式研究新型低共熔溶剂 (DES) 用于萃取 1-己烯/-己烷模型焦化石脑油的有效性。结果表明,氢键受体或氢键供体中的脂肪族或芳香族基团对1-己烯具有较强的亲和力,且含有羟基或芳香族基团的组分具有提供质子的能力,有利于1-己烯的溶解。 。结合基团和基团相互作用贡献模型预测的熔点结果,三种DES,即乙醇胺-环丁砜1:5(DES1)、四丁基溴化铵-双[2-(2-羟基乙氧基)乙基]醚1:4最终制备了(DES2)和甲基三苯基溴化鏻-三甘醇1:4(DES3)。然后,利用量子化学计算研究了DES和1-己烯/-己烷之间的分离机制,证明了由DES和1-己烯之间的色散吸引力主导的单中心和多中心混合弱氢键。最后,通过液-液平衡实验考察了DES的类型、组成和温度对分离性能的影响,结果表明DES1既具有高的1-己烯分配系数,又具有1-己烯/-的选择性。己烷,以及良好的循环稳定性。这项工作表明新型DES可以作为传统有机溶剂和离子液体的替代溶剂,并为焦化石脑油中烯烃和烷烃的分离提供思路。













































 京公网安备 11010802027423号
京公网安备 11010802027423号