Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Ceo2/Cus Nanoplates Electroreduce Co2 to Ethanol with Stabilized Cu+ Species
Small ( IF 13.0 ) Pub Date : 2023-06-03 , DOI: 10.1002/smll.202303099
Zi Yang 1 , Deguang Ji 1 , Zhi Li 1 , Zidong He 1 , Yang Hu 1 , Jie Yin 1 , Yichao Hou 1 , Pinxian Xi 1, 2 , Chun-Hua Yan 1, 3
Small ( IF 13.0 ) Pub Date : 2023-06-03 , DOI: 10.1002/smll.202303099
Zi Yang 1 , Deguang Ji 1 , Zhi Li 1 , Zidong He 1 , Yang Hu 1 , Jie Yin 1 , Yichao Hou 1 , Pinxian Xi 1, 2 , Chun-Hua Yan 1, 3
Affiliation
|
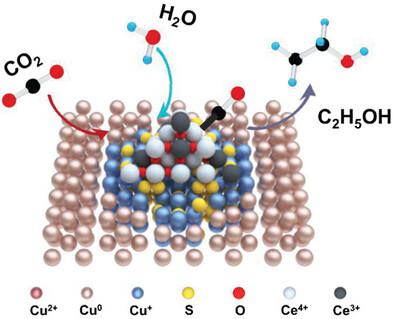
Copper-based electrocatalysts effectively produce multicarbon (C2+) compounds during the electrochemical CO2 reduction (CO2RR). However, big challenges still remain because of the chemically unstable active sites. Here, cerium is used as a self-sacrificing agent to stabilize the Cu+ of CuS, due to the facile Ce3+/Ce4+ redox. CeO2-modified CuS nanoplates achieve high ethanol selectivity, with FE up to 54% and FEC2+ ≈ 75% in a flow cell. Moreover, in situ Raman spectroscopy and in situ Fourier-transform infrared spectroscopy indicate that the stable Cu+ species promote CC coupling step under CO2RR. Density functional theory calculations further reveal that the stronger *CO adsorption and lower CC coupling energy, which is conducive to the selective generation of ethanol products. This work provides a facile strategy to convert CO2 into ethanol by retaining Cu+ species.
中文翻译:

Ceo2/Cus 纳米板用稳定的 Cu+ 物质将 Co2 电还原为乙醇
铜基电催化剂在电化学CO 2还原(CO 2 RR)过程中有效地产生多碳(C 2+ )化合物。然而,由于化学不稳定的活性位点,仍然存在巨大的挑战。这里,由于容易进行Ce 3+ /Ce 4+氧化还原,所以使用铈作为自牺牲剂来稳定CuS的Cu +。CeO 2修饰的CuS纳米板实现了高乙醇选择性,在流通池中FE高达54%,FE C2+ ≈ 75%。此外,原位拉曼光谱和原位傅立叶变换红外光谱表明,稳定的Cu +物质促进了CO 2 RR下的C → C偶联步骤。密度泛函理论计算进一步揭示* CO吸附能力更强, CC耦合能更低,有利于乙醇产物的选择性生成。这项工作提供了一种通过保留Cu +物质将CO 2转化为乙醇的简便策略。
更新日期:2023-06-03
中文翻译:

Ceo2/Cus 纳米板用稳定的 Cu+ 物质将 Co2 电还原为乙醇
铜基电催化剂在电化学CO 2还原(CO 2 RR)过程中有效地产生多碳(C 2+ )化合物。然而,由于化学不稳定的活性位点,仍然存在巨大的挑战。这里,由于容易进行Ce 3+ /Ce 4+氧化还原,所以使用铈作为自牺牲剂来稳定CuS的Cu +。CeO 2修饰的CuS纳米板实现了高乙醇选择性,在流通池中FE高达54%,FE C2+ ≈ 75%。此外,原位拉曼光谱和原位傅立叶变换红外光谱表明,稳定的Cu +物质促进了CO 2 RR下的C → C偶联步骤。密度泛函理论计算进一步揭示* CO吸附能力更强, CC耦合能更低,有利于乙醇产物的选择性生成。这项工作提供了一种通过保留Cu +物质将CO 2转化为乙醇的简便策略。

































 京公网安备 11010802027423号
京公网安备 11010802027423号