European Journal of Cell Biology ( IF 4.5 ) Pub Date : 2023-06-02 , DOI: 10.1016/j.ejcb.2023.151326 Zorica Nakevska 1 , Toshifumi Yokota 2
|
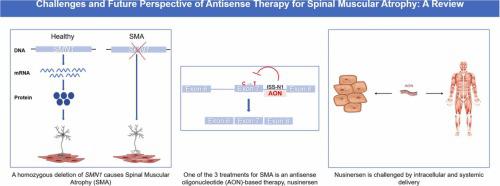
Spinal muscular atrophy (SMA), the most common genetic cause of infantile death, is caused by a mutation in the survival of motor neuron 1 gene (SMN1), leading to the death of motor neurons and progressive muscle weakness. SMN1 normally produces an essential protein called SMN. Although humans possess a paralogous gene called SMN2, ∼90% of the SMN it produces is non-functional. This is due to a mutation in SMN2 that causes the skipping of a required exon during splicing of the pre-mRNA. The first treatment for SMA, nusinersen (brand name Spinraza), was approved by the FDA in 2016 and by the EMU in 2017. Nusinersen is an antisense oligonucleotide-based therapy that alters the splicing of SMN2 to make functional full-length SMN protein. Despite the recent advancements in antisense oligonucleotide therapy and SMA treatment development, nusinersen is faced with a multitude of challenges, such as intracellular and systemic delivery. In recent years, the use of peptide-conjugated phosphorodiamidate morpholino oligomers (PPMOs) in antisense therapy has gained interest. These are antisense oligonucleotides conjugated to cell-penetrating peptides such as Pips and DG9, and they have the potential to address the challenges associated with delivery. This review focuses on the historic milestones, development, current challenges, and future perspectives of antisense therapy for SMA.
中文翻译:

反义疗法治疗脊髓性肌萎缩症的挑战和未来前景:综述
脊髓性肌萎缩症(SMA)是婴儿死亡最常见的遗传原因,是由运动神经元1基因( SMN1)的存活突变引起的,导致运动神经元死亡和进行性肌肉无力。SMN1通常会产生一种称为 SMN 的必需蛋白质。尽管人类拥有一种名为SMN2的旁系同源基因,但它产生的 SMN 约 90% 是无功能的。这是由于SMN2中的突变导致前 mRNA 剪接过程中所需外显子的跳过。第一种 SMA 治疗药物 nusinersen(商品名 Spinraza)于 2016 年获得 FDA 批准,并于 2017 年获得 EMU 批准。Nusinersen 是一种基于反义寡核苷酸的疗法,可改变SMN2的剪接制备功能性全长 SMN 蛋白。尽管反义寡核苷酸疗法和 SMA 治疗开发最近取得了进展,但 nusinersen 仍面临着许多挑战,例如细胞内和全身递送。近年来,肽缀合的磷酸二酰胺吗啉寡聚物(PPMO)在反义治疗中的应用引起了人们的兴趣。这些是与 Pips 和 DG9 等细胞穿透肽缀合的反义寡核苷酸,它们有潜力解决与递送相关的挑战。本综述重点关注 SMA 反义疗法的历史里程碑、发展、当前挑战和未来前景。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号