当前位置:
X-MOL 学术
›
Org. Process Res. Dev.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Improved Synthetic Process of Baloxavir Marboxil Intermediate 3-Benzyloxy-4-oxo-4H-pyran-2-carboxylic Acid
Organic Process Research & Development ( IF 3.1 ) Pub Date : 2023-06-02 , DOI: 10.1021/acs.oprd.2c00387 Kongcheng Huang 1, 2 , Jianwu Lu 2, 3 , Xiaoxu Wang 2 , Yinfei Shi 2 , Kaimike Yang 4 , Shun Yuan 2 , Yinquan Wang 2 , Han Sun 2 , Yuebin Liu 2 , Xun Sun 3 , Taizhi Wu 2
Organic Process Research & Development ( IF 3.1 ) Pub Date : 2023-06-02 , DOI: 10.1021/acs.oprd.2c00387 Kongcheng Huang 1, 2 , Jianwu Lu 2, 3 , Xiaoxu Wang 2 , Yinfei Shi 2 , Kaimike Yang 4 , Shun Yuan 2 , Yinquan Wang 2 , Han Sun 2 , Yuebin Liu 2 , Xun Sun 3 , Taizhi Wu 2
Affiliation
|
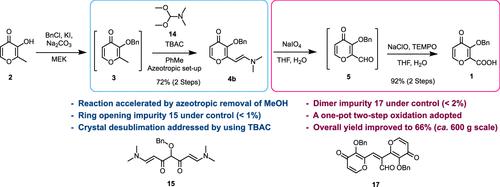
The article presents a process research study of 3-benzyloxy-4-oxo-4H-pyran-2-carboxylic acid (1), a crucial intermediate in the synthesis of baloxavir marboxil. The original four-step sequence exhibited various limitations, such as low yield (43%), the use of problematic solvents, a large excess of costly reagents, safety concerns, and impurity control difficulties. To address these challenges, process optimization was carried out to achieve the desired goals for large-scale industrial production. Optimization of the enamine 4b synthesis was performed, resulting in the development of an azeotropic removal method for the byproduct methanol. This improvement led to minimized ring-opening impurity 15 and a significant reduction in the consumption of the condensation reagent. Furthermore, a simplified one-pot two-step oxidation sequence was devised to streamline the process for the synthesis of compound 1. This optimization involved controlling the formation of hydrolytic impurity 16 and its downstream aldol condensation form 17. The optimized process was successfully demonstrated on a 600 g scale with a product purity of 99.9%, and the overall yield was substantially improved, rising from 43 to 66%.
中文翻译:

Baloxavir Marboxil中间体3-苄氧基-4-氧代-4H-吡喃-2-甲酸的合成工艺改进
本文介绍了 3-苄氧基-4-氧代-4 H-吡喃-2-羧酸 ( 1 )的工艺研究,该酸是 baloxavir marboxil 合成中的关键中间体。最初的四步序列表现出各种局限性,例如产率低(43%)、使用有问题的溶剂、大量过量的昂贵试剂、安全问题和杂质控制困难。为了应对这些挑战,进行了工艺优化,以实现大规模工业生产的预期目标。对烯胺4b合成进行了优化,从而开发了副产物甲醇的共沸去除方法。这一改进最大限度地减少了开环杂质15并显着减少缩合试剂的消耗。此外,设计了简化的一锅两步氧化序列以简化化合物1的合成过程。该优化涉及控制水解杂质16的形成及其下游羟醛缩合形式17。优化后的工艺在600g规模上成功验证,产品纯度达到99.9%,总体收率大幅提高,从43%上升到66%。
更新日期:2023-06-02
中文翻译:

Baloxavir Marboxil中间体3-苄氧基-4-氧代-4H-吡喃-2-甲酸的合成工艺改进
本文介绍了 3-苄氧基-4-氧代-4 H-吡喃-2-羧酸 ( 1 )的工艺研究,该酸是 baloxavir marboxil 合成中的关键中间体。最初的四步序列表现出各种局限性,例如产率低(43%)、使用有问题的溶剂、大量过量的昂贵试剂、安全问题和杂质控制困难。为了应对这些挑战,进行了工艺优化,以实现大规模工业生产的预期目标。对烯胺4b合成进行了优化,从而开发了副产物甲醇的共沸去除方法。这一改进最大限度地减少了开环杂质15并显着减少缩合试剂的消耗。此外,设计了简化的一锅两步氧化序列以简化化合物1的合成过程。该优化涉及控制水解杂质16的形成及其下游羟醛缩合形式17。优化后的工艺在600g规模上成功验证,产品纯度达到99.9%,总体收率大幅提高,从43%上升到66%。































 京公网安备 11010802027423号
京公网安备 11010802027423号